- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Study: Acne a Common Side Effect of Atopic Dermatitis Treatment
Author(s):
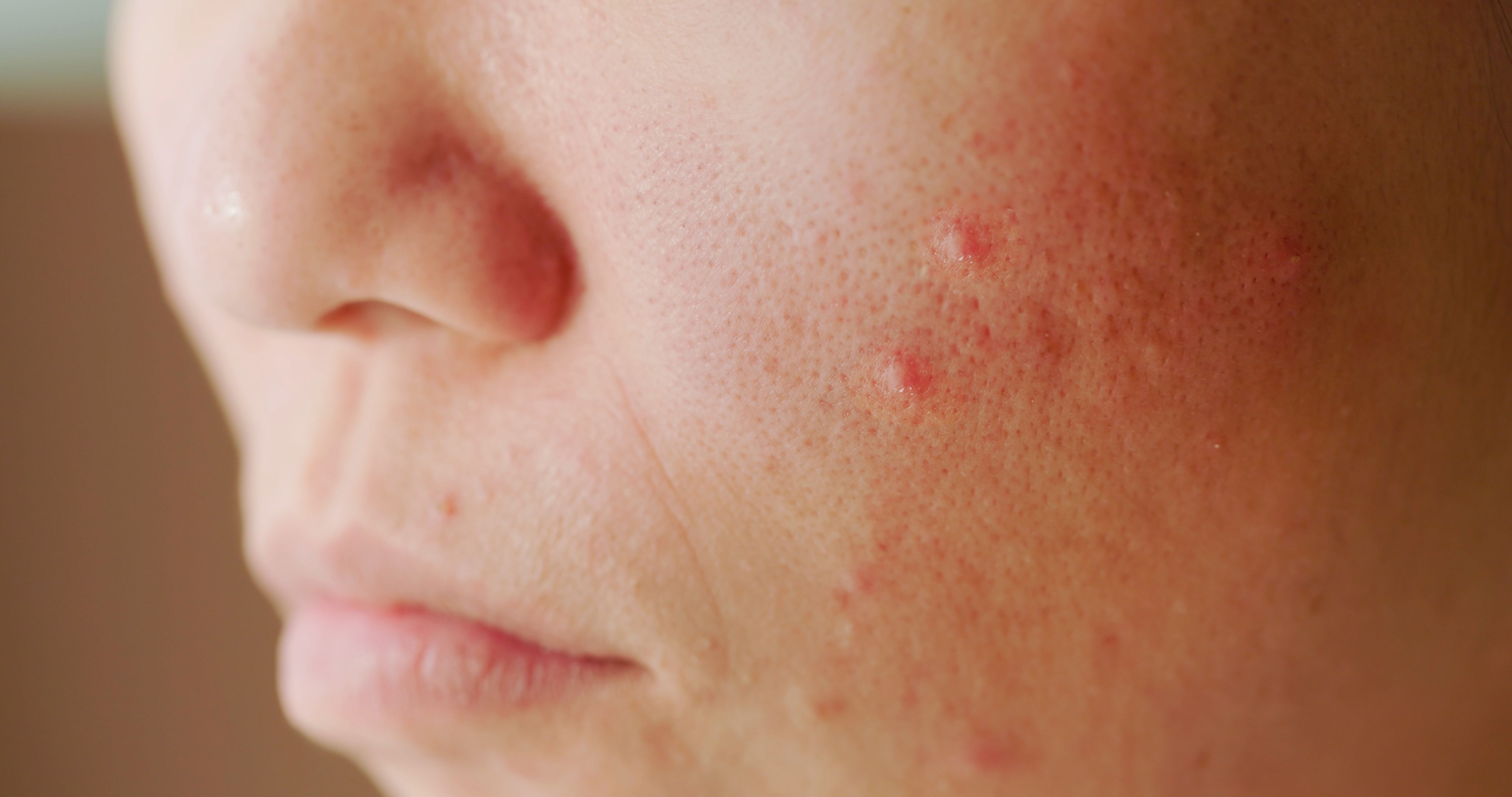
Acne was mild to moderate in severity and successfully managed with topical or oral treatments.
As part of the multicenter, double-blind trial Rising Up (NCT03661138) in Japan, investigators found that patients given upadacitinib for treatment of moderate-to-severe atopic dermatitis (AD) had a higher incidence of acne than patients given placebo.1 The study found mild to moderate acne was dose-dependent and managed with standard treatments.1 Several studies indicate that acne is a common adverse event with all Janus kinase (JAK) inhibitor treatments.2
ryanking999/AdobeStock
In the current study, overall mean age of participants was 35.6 years and they exhibited moderate to severe AD as assessed by the Eczema Area and Severity Index, validated Investigator Global Assessment for Atopic Dermatitis, body surface area affected, and the Worst Pruritis Numerical Rating Scale with symptoms for at least 3 years.
Rising Up was a randomized trial of 272 individuals conducted at 43 sites in Japan to assess the safety and efficacy of upadacitinib for the treatment of moderate-to-severe AD. Patients participated in a 35-day screening period, a 16-week treatment period, a 36-week blinded extension period from weeks 16 to 52, and an open-label extension period from weeks 52 to 160 of treatment. Investigators continued to follow patients for 30 days after the extension period. In an earlier analysis, the investigators found upadacitinib showed “durable long-term efficacy” in adults and adolescents with moderate to severe AD.3
Patients were randomly assigned to receive upadacitinib 15 mg once a day, 30 mg once a day, or placebo. All groups received treatment in combination with concomitant topical corticosteroids. Following the 16-week treatment period, patients given placebo were re-randomized to either 15 mg or 30 mg upadacitinib. Use of concomitant topical medication was optional after 16 weeks.
Investigators evaluated the incidence, severity, duration, clinical characteristics, and management of treatment-emergent acne events from baseline to week 16 and again at the 52-week cutoff point. The National Cancer Institute Common Terminology Criteria for Adverse Events version 5.0 was used to assess acne severity.
Including patients who received placebo at baseline and re-randomized at 16 weeks, a total of 133 patients received 15 mg upadacitinib and 136 patients received 30 mg upadacitinib. A total of 24 patients discontinued the trial.
In the first 16 weeks of the study, 13.2% of patients receiving upadacitinib 15 mg experienced acne, 19.8% on the 30 mg dose experienced acne, and 5.6% of those receiving placebo experienced acne. The cases were mild to moderate in severity. Adults 18 to less than 30 years old had the highest incidence of acne.
At 52 weeks, 17.3% and 32.4% of patients receiving upadacitinib 15 mg and 30 mg, respectively, experienced acne, with most cases occurring on the face. The average time to onset of acne was similar among both doses. Of the 80 acne events that began by week 52, 33 had resolved by the cutoff date.
Predisposing factors contibuting to development of acne in both groups were the use of concomitant medications associated with acne, family history of acne, and a medical history of acne. The authors did not observe a pattern to time of acne onset.
“Although acne is one of the most common treatment-emergent adverse events seen with upadacitinib treatment in adolescents and adults with moderate-to-severe AD, the events are readily manageable with standard acne treatment,” Hayashi et al concluded. “Most physicians considering upadacitinib for the treatment of AD are likely to have experience with acne management, so no precautionary measures are necessary.”
References
- Hayashi N, Ikeda M, Liu J, et al. Acne among Japanese patients with atopic dermatitis receiving upadacitinib in the phase 3 Rising Up study. Dermatol Ther (Heidelb). 2023;13:1817–1830. https://doi.org/10.1007/s13555-023-00961-9
- Lee SD, Ahn HJ, Shin MK. A case series of acne following Janus kinase inhibitors in patients with atopic dermatitis. JAAD Case Rep. 2022;30:11-16. Published 2022 Oct 11. doi:10.1016/j.jdcr.2022.09.029
- Katoh N, Ohya Y, Murota H. et al. Safety and efficacy of upadacitinib for atopic dermatitis in Japan: 2-year interim results from the phase 3 Rising Up study. Dermatol Ther (Heidelb). 2023;13:221–234. https://doi.org/10.1007/s13555-022-00842-7






