- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Severe Hemorrhage from Infantile Hemangioma
Author(s):
A 5-month-old girl with a large scalp infantile hemangioma (IH), present since 6 weeks of age, is evaluated in the emergency department for lethargy and pallor.
The case
A 5-month-old girl with a large scalp infantile hemangioma (IH), present since 6 weeks of age, was evaluated in the emergency department for lethargy and pallor. Two days prior, the patient’s mother witnessed significant bleeding from the tumor that resolved spontaneously.
History and examination
The patient had been on propranolol 2 mg/kg/day for 2 months, managed by an outside provider. On exam, a segmental, mixed-type IH measuring 112.5 cm2 was evident on the right temporoparietal and occipital scalp, with foci of ulceration and hemorrhagic crust.
Laboratory and imaging studies
Laboratory results showed that the patient’s hemoglobin was 5.7 g/dL. Her platelet count and coagulation studies were normal. Magnetic resonance imaging (MRI) of the brain and magnetic resonance angiography (MRA) of the head and neck revealed an intensely enhancing mass fed by numerous branches of the internal and external carotid and vertebral arteries. The tumor completely encased the right internal carotid artery and internal jugular vein without significant narrowing and infiltrated the right paraspinal muscles and parapharyngeal, carotid, retropharyngeal, and masticator spaces. No additional vascular anomalies were identified other than an aberrant right subclavian artery arising distal to the left subclavian, possibly a normal variant. Cardiology did not recommend routine monitoring based on this finding. There were no additional findings concerning for PHACE syndrome (posterior fossa anomalies, hemangioma, arterial lesions, cardiac abnormalities/coarctation of the aorta, eye anomalies).
Treatment and patient outcome
Figure 1
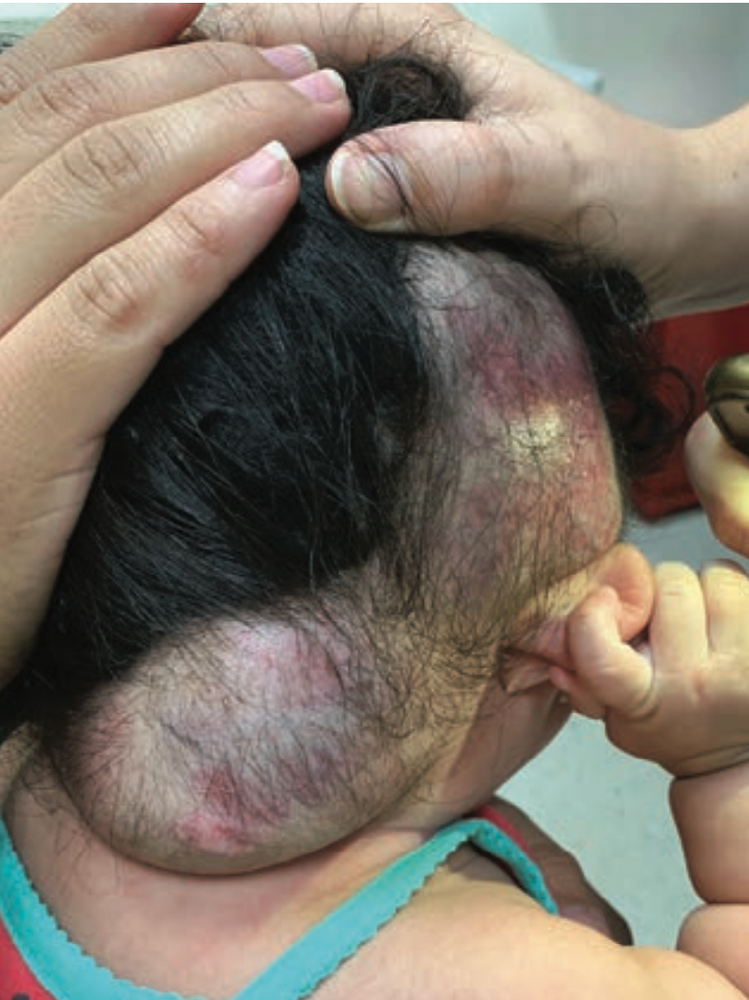
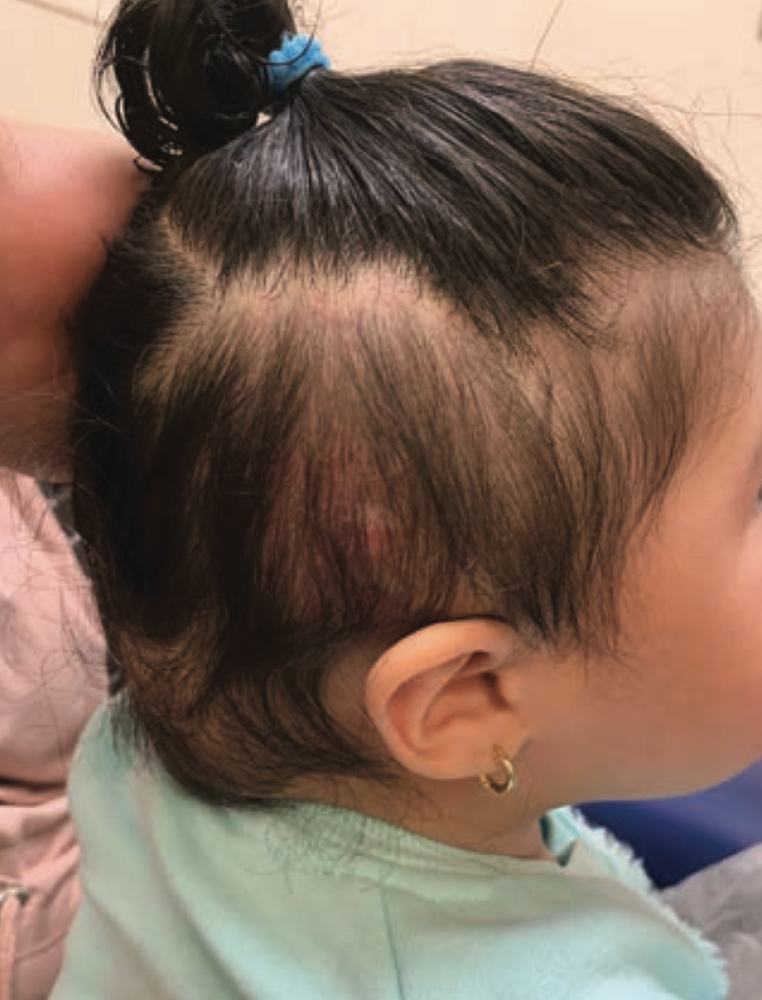
The patient was transfused with 15 cc/kg of packed red blood cells. Propranolol was increased to 3 mg/ kg/day and prednisolone 1 mg/kg/ day was initiated. The lesion responded well to treatment and, after 1 month, had decreased in size without recurrent hemorrhage (Figure 1). Figure 2 depicts the lesion 6 months following hemorrhage and demonstrates further reduction in size.
Figure 2
Differential diagnosis
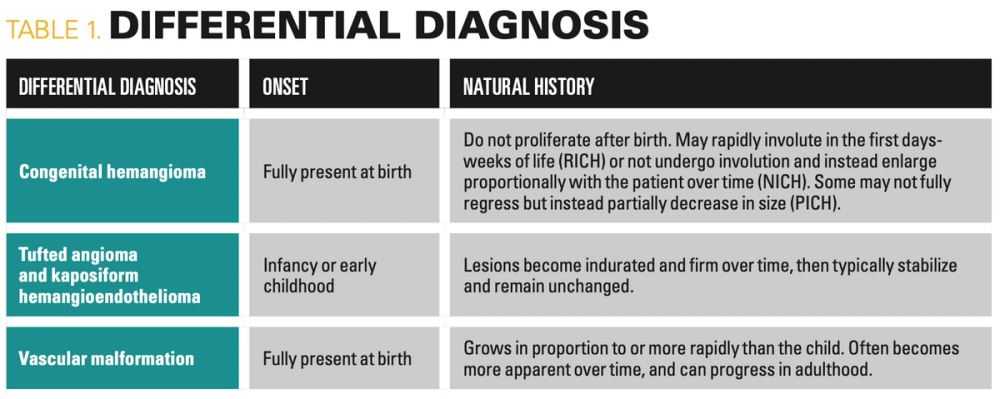
Given the history and morphology of the patient’s tumor, IH was the most likely diagnosis. However, certain differential diagnoses may be considered in an infant with a similar lesion. Onset and natural history are the most important factors distinguishing IHs from other vascular anomalies of infancy (Table 1). Congenital hemangiomas are present at birth and appear as violaceous or pink vascular plaques or exophytic masses with peripheral pallor, typically on the head, neck, or limbs. They can be divided into rapidly involuting congenital hemangiomas (RICH), noninvoluting congenital hemangiomas (NICH), and partially involuting congenital hemangiomas (PICH) based on their clinical course. Because proliferation of these tumors occurs in utero, congenital hemangiomas are fully formed at birth and do not typically grow postnatally, unlike IHs, which characteristically undergo self-limited proliferation followed by spontaneous involution. Tufted angiomas (TAs) and kaposiform hemangioendotheliomas (KHE) are rare vascular tumors that fall under the same neoplastic spectrum and may clinically resemble IHs. TAs appear as indurated, dusky to red plaques or nodules with ill-defined borders, and KHEs present as purpuric subcutaneous masses. TAs and KHEs typically manifest in the first few years of life on the trunk or extremities and, in contrast to IHs, they rarely involve craniofacial areas and do not regress over time. Of note, KHE, and less commonly TA, can be complicated by Kasabach-Merritt phenomenon, which is a life-threatening condition characterized by consumption coagulopathy and thrombocytopenia. Vascular malformations are structural anomalies comprised of capillaries, arteries, veins, and/or lymphatic vessels. They may appear morphologically similar to deep IHs, but are commonly present at birth, grow proportionally with or more rapidly than the child, do not spontaneously regress and can progress in adulthood. We diagnosed this as a case of exceedingly rare, life-threatening hemorrhage from a large IH on the scalp.
Table 1
Discussion
IHs are the most common vascular tumors in infants and become clinically apparent within the first days to months of life. Approximately half appear on the head and neck. They are classified by pattern: localized (focal), segmental (large lesions spanning a linear/geographic territory), or multifocal, as well as by type: superficial (bright red papule/plaque), deep (skin-colored to blue nodule), or mixed (both superficial and deep components). IHs classically undergo a proliferative phase, which is most rapid in the first several months and can last up to 1 year. This is followed by a gradual involution phase, with most involution occurring by 3 to 4 years of age. Thicker hemangiomas leave behind fibrofatty residue that may be amenable to excision, but scarring can be significant. American Academy of Pediatrics (AAP) guidelines exist to help pediatricians identify high-risk IH that require early treatment with propranolol.1
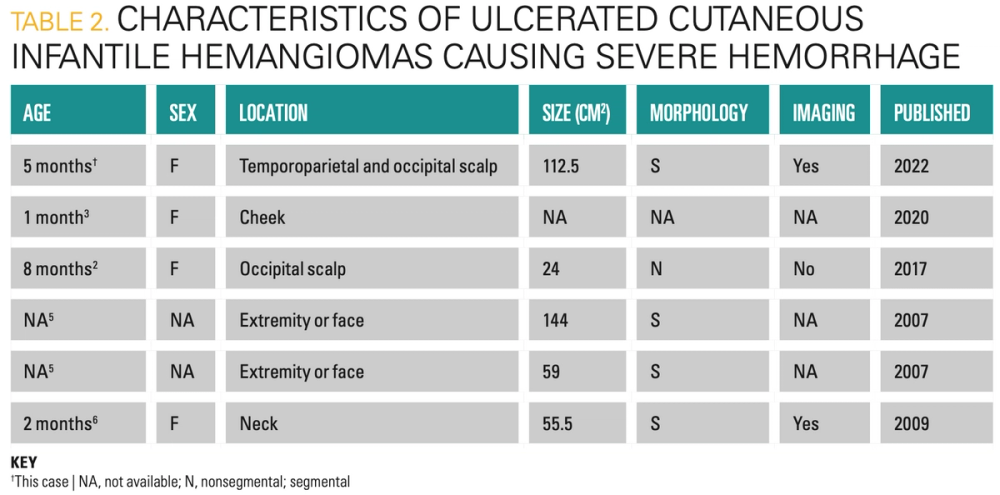
Ulceration occurs in 10% to 25% of IH,2,3 usually during the proliferative phase when demand surpasses blood supply to proliferating capillaries. Mild bleeding is common in ulcerated IH but is rarely clinically significant.4 Life-threatening hemorrhage is exceedingly rare, with only 5 other reported cases in the literature, 4 of which required transfusion (Table 2).2,3,5,6 Most occurred in large, bulky mixed-type IHs, known to have increased risk of ulceration, severe bleeding, and a prolonged proliferative phase.5 These IHs likely had numerous large or anomalous feeder vessels more readily damaged by minor trauma or ulceration. Imaging with arteriography may be helpful in further characterizing the extent of feeder vessels that supply these lesions and may aid in risk stratification for potential hemorrhage. In 1 case of a neck IH, arteriography demonstrated feeders from an aberrant collection of vessels from the thyrocervical trunk, subclavian, external carotid, and vertebral arteries.6 MRA of our patient’s IH demonstrated numerous feeders from the internal carotid, external carotid, and vertebral arteries. The lesion also extended into internal spaces and encased critical vessels. Theoretical complications include internal hemorrhage, serious infection, and massive stroke. The extent of the other reported IHs on imaging was not described, possibly due to inconsistent imaging prior to 2016 PHACE syndrome screening guidelines.7
Table 2
A subset of bulky mixed-type IHs are at a small but serious risk of hemorrhage and should counsel families accordingly. Imaging with MRI and MRA should be performed of large (> 5 cm), bulky, segmental, mixed-type IHs of the head and neck as soon as possible to characterize blood supply, anatomic boundaries, and complication risk.1 AAP guidelines help identify high-risk hemangiomas that require early treatment with propranolol.1 Life-threatening hemorrhage from IHs warrants further study to optimize risk stratification.
References:
1. Krowchuk DP, Frieden IJ, Mancini AJ, et al. Clinical practice guideline for the management of infantile hemangiomas. Pediatrics. 2019;143(1):e20183475. doi:10.1542/peds.2018-3475
2. Charny JW, Moon AT, Treat JR. Scalp infantile hemangioma complicated by life-threatening bleeding. Pediatr Dermatol. 2017;34(4):473-475. doi:10.1111/pde.13171
3. Yildirimcakar D, Demirsoy U, Azizoglu M, Corapcioglu F. Evaluation of clinical properties and treatment responses of infantile hemangioma. J Drugs Dermatol. 2020;19(12):1156-1165. doi:10.36849/JDD.2020.5009
4. Darrow DH, Greene AK, Mancini AJ, Nopper AJ. Diagnosis and management of infantile hemangioma. Pediatrics. 2015;136(4):e1060-1104. doi:10.1542/peds.2015-2485
5. Chamlin SL, Haggstrom AN, Drolet BA, et al. Multicenter prospective study of ulcerated hemangiomas. J Pediatr. 2007;151(6):684-689.e1. doi:10.1016/j.jpeds.2007.04.055
6. Connelly EA, Viera M, Price C, Waner M. Segmental hemangioma of infancy complicated by life-threatening arterial bleed. Pediatr Dermatol. 2009;26(4):469-472. doi:10.1111/j.1525-1470.2009.00955.x
7. Garzon MC, Epstein LG, Heyer GL, et al. PHACE syndrome: consensus-derived diagnosis and care recommendations. J Pediatr. 2016;178:24-33.e22. doi:10.1016/j.jpeds.2016.07.054
This content was originally published by our sister brand, Contemporary Pediatrics.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.





