- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Ruxolitinib, Educational Initiatives for Managed Care Professionals Play Crucial Role in Optimizing Care in Atopic Dermatitis
Author(s):
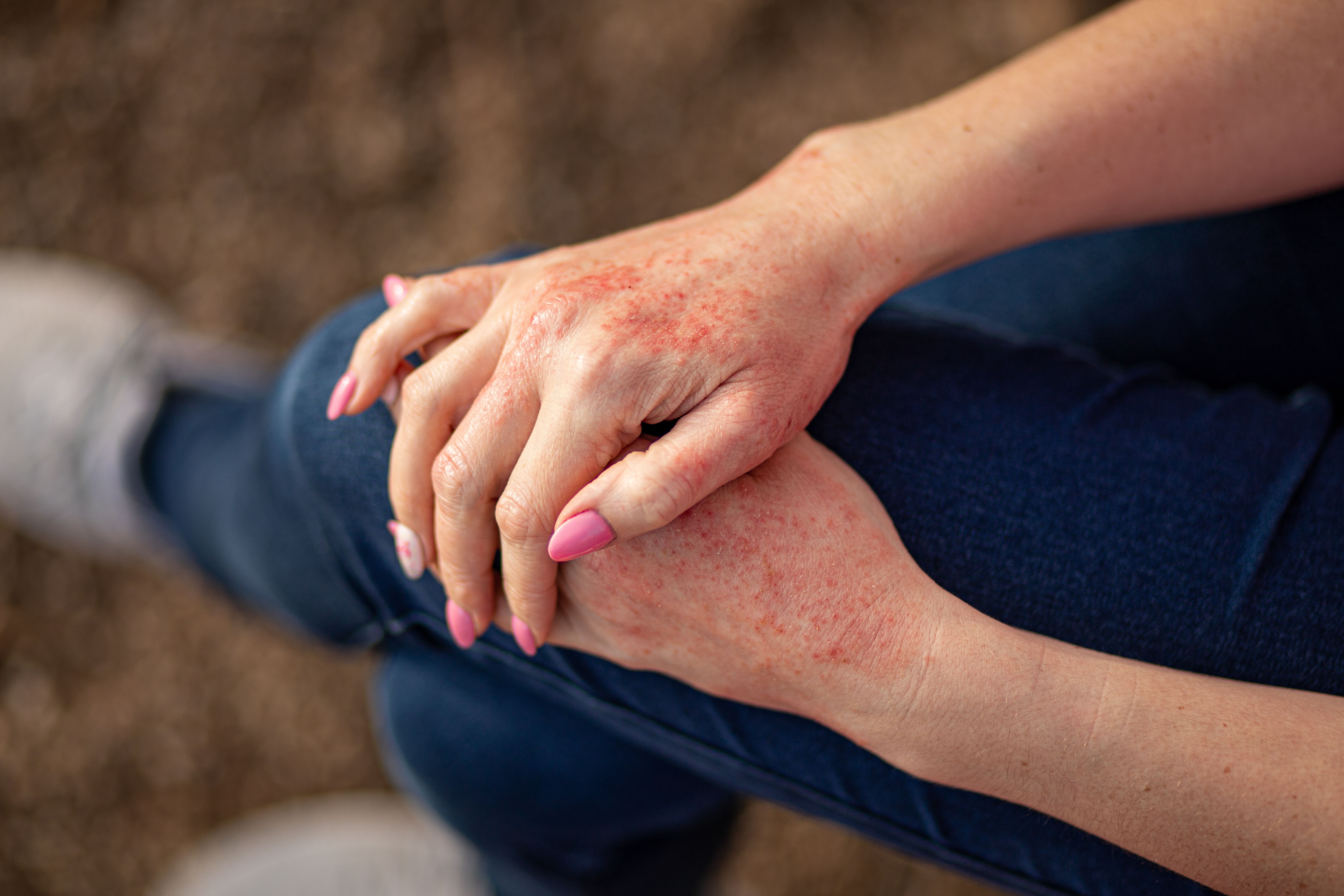
Posters from the Academy of Managed Care Pharmacy annual meeting featured studies on atopic dermatitis.
A section of the poster hall at the Academy of Managed Care Pharmacy (AMCP) 2024 annual meeting, held in New Orleans, Louisiana, was dedicated to research and initiatives throughout the field of atopic dermatitis (AD). Among these posters, Jinan Liu, MD, PhD, and colleagues showcased data on the impact of ruxolitinib cream on corticosteroid and biologic use in patients with AD, and Michele Guadalupe, MPH, and coauthors detailed results from the implementation of an educational initiative on managed care professionals’ understanding of AD.
The first presentation, titled “Does Ruxolitinib Cream Reduce Corticosteroid and Biologic Use in Patients With a History of Moderate to Severe Atopic Dermatitis?,” assessed treatment patterns following the initiation of ruxolitinib cream in patients with moderate to severe AD.1 Specifically, this investigation targeted patients who had prior experience with phototherapy, topical corticosteroids (TCS), or systemic therapies (advanced therapies).
Eligible patients were retrospectively identified with claims data that were gathered from the Healthcare Integrated Research Database (HIRD). Individuals were included if their first claim for ruxolitinib cream came between October 2021 and July 2022 and if they had a documented history with advanced therapies at baseline. Baseline (preindex) was 6 months prior to an individual’s index date (their first claim) and follow-up occurred in the 6 months following.
In total, 749 patients with AD were included in this study, all of whom were 12 years or older (41.5 years on average). Prior to their first claim for ruxolitinib cream, 535 patients (71.4%) had received a form of topical therapy for AD. For 310 patients (41.4%), a very potent TCS was administered, and in 573 (76.5%), a systemic therapy.
Throughout the postindex follow-up period, an average of 1.7 claims for ruxolitinib cream were filed per patient. There were 551 patients (73.6%) who were not administered a new class of AD treatment after the ruxolitinib cream was administered. Researchers saw an overall decrease of 50% in claims for TCS, topical calcineurin inhibitors (a common first-line therapy in AD), and phosphodiesterase-4 inhibitors (another first-line therapy) compared with the preindex period.
Additionally, researchers witnessed oral corticosteroid use fall from 44.1% (n = 330) to 20.7% (n = 155) during the postindex follow-up period, and the average prednisone-equivalent dose cumulatively fell by almost 50% (163.0 mg to 82.5 mg).
During the preindex period, 298 patients had claims for biologics, and patient-continuation numbers dropped by 17.4% (n = 52) throughout the follow-up period. Of the total 451 patients who were naive to biologics preindex, 88.5% (n = 399) continued without biologics throughout the follow-up period.
Considering these findings, Liu et al concluded that ruxolitinib cream could, in the shor -term, limit the use of biologics and/or topical and oral corticosteroids.
“I think this is exactly what we expected...ruxolitinib cream is positioned to betray the generics—the cheap drugs—and also the systemic therapies,” Liu commented when asked about any surprising findings that arose over the course of study. Thinking toward the future of this research, he added, “So this is the data we were able to produce with the national payer, Anthem Blue Cross Blue Shield. Next step, we will replicate the same analysis with a different payer, UnitedHealthcare. And also, currently we only have 6-month follow-up period data; we’re going to expand that follow-up period to 1 year...to show exactly the same benefit of initiation over our screens.”
The other investigation, titled “Impact of a Multi-Year Initiative for Managed Care Professionals to Increase Their Understanding of Atopic Dermatitis (AD),” aimed to address knowledge gaps in the field that may negatively impact the quality of care experienced by and accessible to patients with AD.2
Multiple continuing education activities were developed by the National Eczema Association (NEA) and Impact Education, LLC (IMPACT) for managed care professionals. The initiatives were informed by best practices that were previously created via collaboration among community members, payers, and providers.
A total of 3 webinars series were conducted, in which 1000 managed care professionals across the US participated. In total, 78% of states were represented, and the learning initiatives were anticipated to beneficially impact the care of over 250,000 patients with AD.
Results showed that, in the 2021-2022 webcast series, there was a percentage-point increase of 60 in learners’ ability to recognize itch as AD’s most burdensome symptom (20% to 80%). Additionally, in the 2022-2023 webcast series, 57% and 58% of learners correctly recognized the widespread incidence of AD in US children (10 million) and the increased likelihood of suicide attempts (36%) in US adults with AD, respectively, compared with only 15% and 13% prior to the initiative. Furthermore, learners had a statistically significant change in confidence levels when it came to being able to identify the multifaceted burdens linked to living with moderate to severe AD, as well as the correlations between AD and mental health, metabolic, autoimmune, and cardiovascular conditions (P < .01). These findings demonstrated the beneficial impact that education can have, which subsequently can enhance their abilities to perform best practices for their patients.
As the team of researchers intends to expand the impact of these initiatives, Guadalupe reflected on the implications of their work. “We really wanted to bring payers, providers, and patients together, so that they can have those conversations, so that the providers could share with payers, ‘this is really what atopic dermatitis looks like,’ and really go into that the disease, into the quality of life actors, and bringing in the patients,” Guadalupe commented. She added, “Sometimes language that a payer uses isn't the same that a provider uses.... That's our kind of our misunderstanding here: How do they measure? How do we measure?” With the ability to actually connect and communicate effectively with a payer about a patient’s condition, she continued, meaningful benefits can be achieved in patient care.
References
- Liu J, Desai K, Teng CC, Sturm D, Gandhi M, Stockbower G. Does ruxolitinib cream reduce corticosteroid and biologic use in patients with a history of moderate to severe atopic dermatitis? Poster presented at: Academy of Managed Care Pharmacy 2024; April 15-18, 2024; New Orleans, LA. Presentation L4.
- Waite-Ardini S, Guadalupe M, Begolka WS, Bartolini JL, Casebeer S, Richardson T. Impact of a multi-year initiative for managed care professionals to increase their understanding of atopic dermatitis (AD). Poster presented at: Academy of Managed Care Pharmacy 2024; April 15-18, 2024; New Orleans, LA. Presentation L10.
[This article was originally published by our sister publication, American Journal of Managed Care.]