- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Reviewing the Rosacea Pipeline: A Look at the Last 10 Years
Author(s):
In recognition of Rosacea Awareness Month, Dermatology Times is reviewing research and strides in rosacea treatment over the last decade.
The US Food and Drug Administration (FDA) has approved several therapies for patients with rosacea over the years. From innovations such as azelaic acid 15% gel approved in 2002 to the anticipated approval of Journey Medical Corporation's DFD-29 later this year, research and stamps of approval have led to significant improvements in patient care.
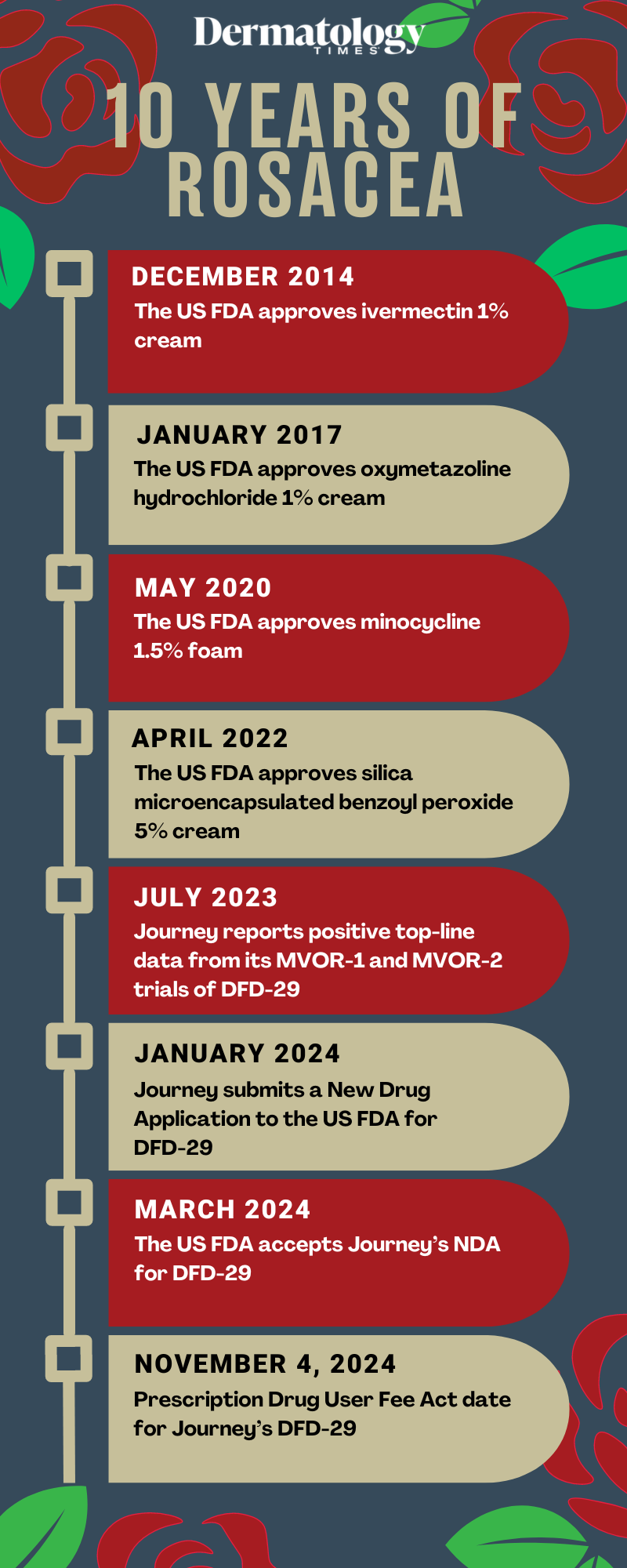
This Rosacea Awareness Month, Dermatology Times is recapping the last 10 years of news in the rosacea drug pipeline.
December 2014: Ivermectin 1% cream is approved by the FDA
Galderma Laboratories announced the FDA approval of Soolantra (ivermectin) cream, 1% for the topical treatment of rosacea in December 2014.1 Clinical studies demonstrated its efficacy, with effects observed as early as week 2 and continuous improvement noted over time. In comparisons with metronidazole 0.75% cream, Soolantra was found to be more effective from week 3 onwards.
Long-term extensions of these studies demonstrated the cream's safety and tolerability for up to 52 weeks. Despite its effectiveness, some users experienced skin burning sensation and irritation.
January 2017: Oxymetazoline hydrochloride 1% cream is approved by the FDA
Allergan announced the approval of Rhofade (oxymetazoline hydrochloride 1% cream) by the US FDA in January 2017 for the topical treatment of persistent facial erythema associated with rosacea in adults.2 The approval was based on 2 clinical studies that evaluated the primary efficacy endpoint on day 29.
In 2 clinical trials, a once-daily application of Rhofade had been proven to reduce persistent facial erythema associated with rosacea through 12 hours. The primary efficacy endpoint had been at day 29, defined as the proportion of patients with at least a 2-grade reduction in erythema from baseline on both the clinician erythema assessment and subject self-assessment measured at various time intervals.
May 2020: Minocycline 1.5% foam is approved by the FDA
On May 29, 2020, Menlo Therapeutics announced that the US FDA had approved Zilxi (minocycline) topical foam, 1.5%, for the treatment of inflammatory lesions of rosacea in adults.3 Zilxi became the first minocycline product of any kind to be approved by the FDA for use in rosacea.
The approval was primarily supported by data from 2 clinical trials involving 1,522 patients aged 18 years and older. In each 12-week multicenter, randomized, double-blind, vehicle-controlled trial, subjects with inflammatory lesions of rosacea had been treated once daily with Zilxi or vehicle. Zilxi had met both co-primary endpoints in each clinical trial, demonstrating statistically significant improvements in inflammatory lesion count and Investigator Global Assessment treatment success.
April 2022: Silica microencapsulated benzoyl peroxide 5% cream is approved by the FDA
On April 25, 2022, Sol-Gel Technologies announced the FDA approval of Epsolay, a proprietary cream formulation of benzoyl peroxide, 5%, for the treatment of inflammatory lesions of rosacea in adults.4
The FDA approval had been supported by data from 2 positive phase 3 randomized, double-blind, multicenter, 12-week clinical trials involving 733 participants. Epsolay had demonstrated superiority to vehicle cream in reducing inflammatory lesions of rosacea, with nearly 70% reduction observed by the end of the 12-week trials compared to 38-46% with vehicle.
July 2023: Journey reports positive top-line data from MVOR-1 and 2 for DFD-29
On July 11, 2023, Journey Medical Corporation announced positive topline results from 2 phase 3 multicenter, randomized, double-blind, parallel-group, active-comparator, and placebo-controlled clinical trials, Minocycline Versus Oracea in Rosacea-1 (“MVOR-1”) and Minocycline Versus Oracea in Rosacea-2 (“MVOR-2”), evaluating minocycline hydrochloride modified release capsules, 40 mg (“DFD-29”) for the treatment of moderate-to-severe papulopustular rosacea in adults.5
DFD-29 demonstrated statistical superiority over both Oracea (doxycycline) capsules and placebo for IGA treatment success in both studies, as well as for the reduction in the total inflammatory lesion count. In both MVOR-1 and MVOR-2 trials, subjects were randomized to treatment with DFD-29, Oracea, or placebo once daily for 16 weeks. The proportion of subjects achieving Investigator’s Global Assessment treatment success in the DFD-29 group was statistically superior to those in Oracea and placebo groups.
January 2024: Journey submits NDA to FDA for DFD-29
Journey announced that the company had submitted a New Drug Application (NDA) to the FDA seeking approval for DFD-29 (minocycline hydrochloride modified release capsules, 40 mg) for the treatment of inflammatory lesions and erythema of rosacea in adults. DFD-29 was being developed in collaboration with Dr. Reddy’s Laboratories Ltd.6
The NDA submission was supported by positive data from Journey's 2 DFD-29 phase 3 clinical trials for rosacea treatment. These trials achieved all co-primary and secondary endpoints, and subjects completed the 16-week treatment with no significant safety issues. DFD-29 demonstrated statistical superiority over both the current standard-of-care treatment, Oracea 40 mg capsules, and placebo for Investigator’s Global Assessment treatment success, as well as the reduction in the total inflammatory lesion count in both studies. On a secondary endpoint related to erythema assessment, DFD-29 showed a statistically significant reduction in Clinician’s Erythema Assessment compared to placebo in both clinical trials.
March 2024: FDA accepts Journey's NDA for DFD-29
On March 18, Journey announced that the FDA had accepted the company’s NDA for DFD-29 for the treatment of inflammatory lesions and erythema of rosacea in adults. The FDA set a Prescription Drug User Fee Act goal date of November 4.
References
- Galderma Laboratories, L.P. News release. Galderma receives FDA approval for novel treatment option for rosacea patients. December 23, 2014. Accessed April 15, 2024. https://www.galderma.com/us/news/galderma-receives-fda-approval-novel-treatment-option-rosacea-patients#:~:text=WORTH%2C%20TEXAS%20%E2%80%93%20December%2023%2C,bumps%20and%20pimples%2C%20of%20rosacea
- Allergan. News release. Allergan announces FDA approval of Rhofade (oxymetazoline hydrochloride) cream, 1% for the topical treatment of persistent facial Eeythema associated with rosacea in adults. January 19, 2017. Accessed April 15, 2024. https://www.prnewswire.com/news-releases/allergan-announces-fda-approval-of-rhofade-oxymetazoline-hydrochloride-cream-1-for-the-topical-treatment-of-persistent-facial-erythema-associated-with-rosacea-in-adults-300393385.html
- Menlo Therapeutics. News release. Menlo Therapeutics receives FDA approval of Zilxi (minocycline) topical foam, 1.5 percent: The first topical minocycline treatment for rosacea. BioSpace. May 29, 2020. Accessed April 15, 2024. https://www.biospace.com/article/releases/menlo-therapeutics-receives-fda-approval-of-zilxi-minocycline-topical-foam-1-5-percent-the-first-topical-minocycline-treatment-for-rosaceazilxi-now-approved-for-inflammatory-lesions-of-rosacea-in-adults/
- Sol-Gel Technologies Ltd. & Galderma. News release. Sol-Gel Technologies and Galderma Announce FDA Approval of Epsolay. GlobeNewswire. April 25, 2022. Accessed April 15, 2024. https://www.globenewswire.com/news-release/2022/04/25/2427977/0/en/Sol-Gel-Technologies-and-Galderma-Announce-FDA-Approval-of-EPSOLAY.html
- Journey Medical Corporation. News release. Journey Medical Corporation announces positive topline data from phase 3 study of novel treatment for rosacea. July 11, 2023. Accessed April 15, 2024. https://ir.journeymedicalcorp.com/new-events/press-releases/detail/47/journey-medical-corporation-announces-positive-topline
- Journey Medical Corporation submits New Drug Application to FDA for DFD-29 to treat rosacea. News release. Journal Medical Corporation. January 5, 2024. Accessed April 15, 2024. https://ir.journeymedicalcorp.com/new-events/press-releases/detail/57/journey-medical-corporation-submits-new-drug-application-to
- Journey Medical Corporation announces US FDA acceptance of new drug application for DFD-29 for the treatment of rosacea. News release. Journey Medical Corporation. March 18, 2024. Accessed April 15, 2024. https://ir.journeymedicalcorp.com/new-events/press-releases/detail/61/journey-medical-corporation-announces-u-s-fda-acceptance
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.