- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Range of reasonable options available for psoriasis control during pregnancy
Chicago — As psoriasis often affects persons during their reproductive years, dermatologists are often faced with decisions regarding management of patients who are pregnant or who want to have a child.
Chicago - As psoriasis often affects persons during their reproductive years, dermatologists are often faced with decisions regarding management of patients who are pregnant or who want to have a child.
The good news for women is that various studies report psoriasis improves in more than 50 percent of patients during pregnancy. In addition, although several commonly used treatments are absolutely contraindicated because they are known teratogens, there are still a number of therapeutic modalities that can be used with reasonable safety to control psoriasis across its entire spectrum of severity, said David M. Pariser, M.D., at the American Academy of Dermatology's Academy '05.

In the realm of topical therapies, corticosteroids, calcipotriene (Dovonex, LEO Pharma A/S), tacrolimus (Protopic, Fujisawa) and pimecrolimus (Elidel, Novartis) can all be offered to treat psoriasis during pregnancy. Each has a pregnancy category C rating.
Cyclosporine and meth-oxsalen (Oxsoralen-Ultra, Valeant) used for PUVA are also rated pregnancy category C, and in general should be avoided whenever possible. Although results from various studies provide no evidence that systemic PUVA is teratogenic or adversely affects infant mortality, its use during pregnancy has been associated with low birthweight, and PUVA is a potential mutagen, Dr. Pariser says.
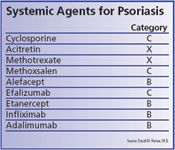
Among the newer biologics that are used to treat psoriasis, efalizumab (Raptiva, Genentech) carries a pregnancy category C rating, while alefacept (Amevive, Biogen), etanercept (Enbrel, Amgen-Wyeth), infliximab (Remicade, Centocor), and adalimumab (Humira, Abbott Immunology) all have category B ratings.
"The amount of clinical experience with these agents varies, but there is no evidence from controlled clinical trials or spontaneous post-marketing reports associating any with an increased risk of miscarriage or congenital malformations," Dr. Pariser says. "Nevertheless, there is some concern regarding their theoretical potential to affect the developing immune system in the fetus, and that is a risk that will need to be assessed through further study."
Among the biologics, the most comprehensive information about use during pregnancy exists for infliximab. Prospectively-collected registry data from patients with Crohn's disease, including more than 2,500 patients who received infliximab, reveals no cases of fetal abnormalities, increased rates of miscarriages or other serious neonatal complications. Corroborating data are also available from smaller retrospective studies of women treated with that drug.
Retrospective analyses have also investigated potential risks of using etanercept during pregnancy without identifying any worrisome signals, and similarly there have been no fetal abnormalities associated with use of efalizumab or alefacept in controlled trials or post-marketing experience. To date there has been the least experience with adalimumab. There were no pregnancies among women treated with that agent during clinical trials, but there have also been no spontaneous reports associating its use with congenital malformations.






