- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Procter & Gamble Issue Voluntary Recall of Certain Old Spice, Secret Products
Author(s):
Procter & Gamble have issued a voluntary recall of specific Old Spice and Secret brand aerosol antiperspirants and Old Spice Below Deck aerosol spray due to benzene detection.
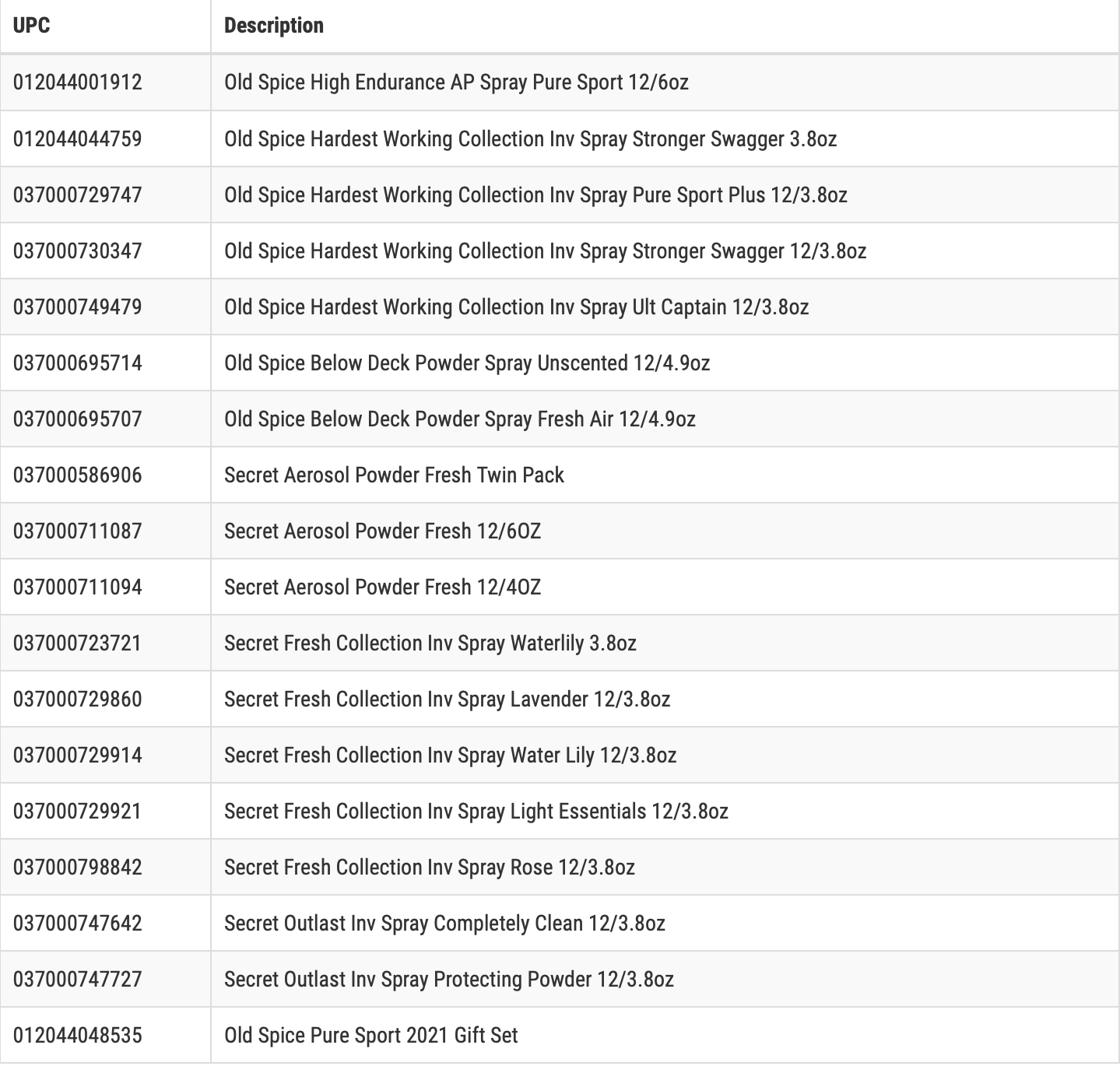
Yesterday, the Procter & Gamble Company (P&G) voluntarily recalled all lots with the expiration date of September 2023 of certain Old Spice and Secret aerosol spray antiperspirants and Old Spice Below Deck aerosol spray products sold in the United States.1 The UPC codes and product names are below for the recalled products. This recall was due to the detection of the carcinogen benzene.
Courtesy of the FDA website.
Benzene is classified as a human carcinogen. Exposure to benzene can occur by inhalation, orally, and through the skin and can result in cancers including leukemia and blood cancer of the bone marrow and blood disorders which can be life-threatening.
Based on exposure modeling and the cancer risk assessments published by the Environmental Protection Agency (EPA) (IRIS database), daily exposure to benzene in the recalled products at the levels detected in the testing would not be expected to cause adverse health consequences, according to the FDA website.
To date, The Procter & Gamble Company has not received any reports of adverse events related to this recall and is conducting this recall out of an abundance of caution.
These products have been distributed across the US through both online and in-stores sales. All other Old Spice and Secret products are not impacted by this issue and may continue to be used as intended, according to the company.
P&G has alerted retailers to remove the products and will also offer reimbursement for consumers who have purchased products impacted by the recall. They advise that consumers should stop using affected products and dispose of them properly.
For more information on how to contact the company please click here.
This news comes after Valisure, a private pharmaceutical company, filed an FDA Citizen Petition on the findings of benzene in several brands and batches of antiperspirant body sprays. The highest contamination was in Old Spice branded body spray. It was found that 54% of the 108 batches from 30 different brands tested by Valisure contained detectable benzene, with some batches containing up to 9 times the emergency FDA limit of 2 parts per million (ppm).
“These findings build upon our now validated discovery of benzene in sunscreens that led to Johnson & Johnson's recall of Neutrogena products, Coppertone's sunscreen recall, and Bayer’s recall of antifungal sprays Tinactin and Lotrimin. In addition, Valisure detected benzene in hand sanitizers which led to ArtNaturals’ recall,” David Light, the founder and CEO of Valisure, commented.
Links to the citizen petition and more information can be found here.
Key findings from the petition are:
- The most contaminated batch, Old Spice branded body spray, contained 17.7 ppm of benzene. If a 5-gram application of this contaminated aerosol is sprayed in a bathroom it could potentially increase the level of benzene to approximately 15 times the EPA-estimated threshold for increased cancer risk.
- Brands in which benzene was detected at 2 ppm or higher include Old Spice, Secret, Tag, Sure, Equate, Suave, Right Guard, and Brut.
- Many petroleum products are used as raw materials or inactive ingredients in consumer healthcare products. Particularly with body sprays, “propellants” like butane, isobutane, propane, and alcohol are commonly used and could potentially be sources of benzene contamination.
- Samples from brands containing significant benzene were analyzed by Yale University’s CBIC analytical laboratory and the contamination was confirmed.
Light did comment on the voluntary recall. "We at Valisure applaud Procter & Gamble for its quick attention to and action on our findings published in our FDA Citizen Petition on benzene contamination in body sprays," he said. "These product contamination issues might be attributed mainly to quality problems starting at the raw materials. These and other issues identified by Valisure strongly underscore the importance of independent testing and its need to be better integrated into an increasingly complex and vulnerable global supply chain."
Light continued. "We hope regulators and manufacturers continue to take further action on body sprays and other products affected by carcinogenic contamination so that consumers do not need to worry about exposure to unnecessary risk."
Reference:
1. P&G issues voluntary recall of specific old spice and secret aerosol spray antiperspirants and old spice below deck aerosol spray products due to detection of benzene. U.S. Food and Drug Administration. Press release. Published November 24, 2021. Accessed November 24, 2021. https://www.fda.gov/safety/recalls-market-withdrawals-safety-alerts/pg-issues-voluntary-recall-specific-old-spice-and-secret-aerosol-spray-antiperspirants-and-old-spice






