- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
PROAK Study: Tirbanibulin Boosts Quality of Life for Patients With Actinic Keratosis
Author(s):
Five days of treatment with the topical therapy measurably improved symptoms and emotional/functional impact for patients with actinic keratosis at week 8.
(Adobe Stock/Henk Vrieselaar)
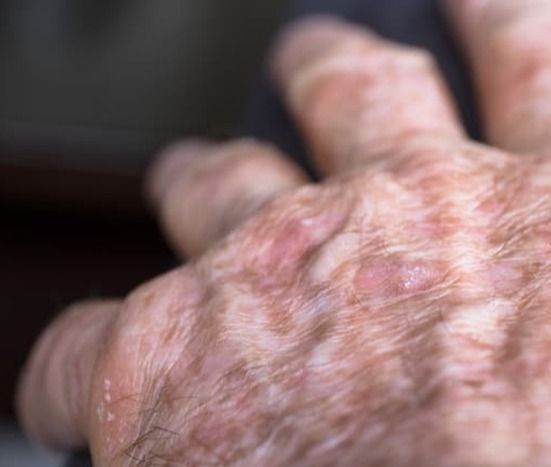
Adult patients with actinic keratosis (AK) who received once-daily tirbanibulin reported a significant reduction in AK burden and an improvement in AK symptoms, as measured by emotional and functional impact, according to the results of the PROAK single-arm prospective cohort study (NCT05260073).
In a poster1 presented at Winter Clinical Miami, held February 17-20, 2023, in Miami, Florida, investigators shared 8-week patient-reported outcomes from a group of adults in community practices across the United States who had been treated with once-daily tirbanibulin (Klisyri; Almirall) for 5 days.
A total of 300 individuals with AK on the face and scalp were enrolled from 32 community practices. In order to assess the safety and efficacy of the study drug, participants completed surveys and clinical assessments at baseline, at 8 weeks, and at 24 weeks.
The 16-item Skindex-16 survey, completed at baseline and at week 8, included 3 domains ranging from symptoms (4 items), emotions (7 items), and functioning (5 items). Investigators scored all items on a 7-point adjectival response scale, with 0 equating to “never bothered” and 6 equating to “always bothered.” Each of the 3 domain scores is then individually calculated on a scale of 0 to 100 with severe impairment due to AKs represented by a higher score. The baseline Skindex-16 scores were 22.30 for symptoms, 38.17 for emotions, and 14.41 for functioning.
The poster comprises data from 290 patients with AK who completed the week 8 study assessment. Participants were majority male (68.62%) with Fitzpatrick skin Type II (71.38%) and a history of skin cancer (61.72%). Patients reported their skin texture at baseline as smooth (47.59%), dry (39.66%), scaly (35.17%), rough (19.66%), bumpy (18.62%), peeling (6.21%), or blistering (0.34%). More than half of participants (56.55%) reported moderate severity of skin photodamage in AK-affected area at baseline, with 20.34% reporting severe skin photodamage in AK-affected area.
All of the 32 community practices included in the study were private, office-based practices that managed a mean of 136.34 patients with AK in a given month and whose clinicians had been practicing dermatology for a mean of 15.66 years.
A total of 77.93% of patients with facial AK lesions and 33.79% of patients with AK lesions on their scalps received treatment with tirbanibulin, with 100% of all participants completing the once-daily dose for the full 5 days. Data from 10 patients were not included in the 8-week analysis; 9 of those individuals were either lost to follow-up or voluntarily withdrew from the study, and 1 patient had missing data.
After 8 weeks of observation, investigators noted a statistically significant (P < 0.0001) decrease in all Skindex-16 domains compared with baseline (symptoms: 8.15, emotions: 13.49, functioning: 4.63). Further, there were no discontinuations of the study drug related to adverse drug reactions and no reports of serious adverse drug reactions as of week 8.
In an exclusive interview with Dermatology Times®, Neal Bhatia, MD, director of clinical dermatology at Therapeutics Clinical Research in San Diego, California, and one of the study investigators, explained some of the findings and what sets tirbanibulin apart from other AK treatment options.
“The outcome is based on the patient experience of local skin reactions, but also the cosmetic outcomes that come from treatment,” he said. “You've had a lot of patients with less erythema, less crusting, and less dermal effects, more superficial effects from the turnover of AKs [actinic keratoses], mainly because the mechanism of action lends itself to apoptosis of the atypical keratinocytes and not necrosis like we've seen with 5-FU [topical 5-fluorouracil] and ingenol mebutate.”
PROAK investigators previously reported clinician-reported outcomes of using tirbanibulin for patients with AK. Overall, clinicians were satisfied that the majority of patients who received once-daily tirbanibulin treatment for 5 days achieved better-looking skin and skin texture by week 8.2
Tirbanibulin was approved3 in December 2020 for the topical treatment of AK on the face or scalp based on data from one of the largest phase 3 clinical study programs conducted for a topical AK treatment, as well as 2 double-blind, vehicle-controlled phase 3 studies (KX01-AK-003 [NCT03285477] and KX01-AK-004 [NCT03285490]).
References:
- Kircik L, Schlesinger T, Armstrong A, et al. Impact of actinic keratosis (ak), as measured by patient-reported ak symptoms, and impact on emotions and functioning (using skindex-16) among patients with ak administered tirbanibulin in real-world community practices across the US (PROAK study). Poster presented at Winter Clinical Miami 2023 meeting; February 17-20, 2023; Miami Beach, FL; Accessed February 14, 2023.
- Bader K. Tirbanibulin improves skin in patients with actinic keratosis. Dermatology Times. Published February 12, 2023. Accessed February 14, 2023. https://www.dermatologytimes.com/view/tirbanibulin-improves-skin-in-patients-with-actinic-keratosis
- Barrett J. FDA approves topical for actinic keratosis. Dermatology Times. Published December 16, 2020. Accessed February 14, 2023. https://www.dermatologytimes.com/view/fda-approves-topical-for-actinic-keratosis






