- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
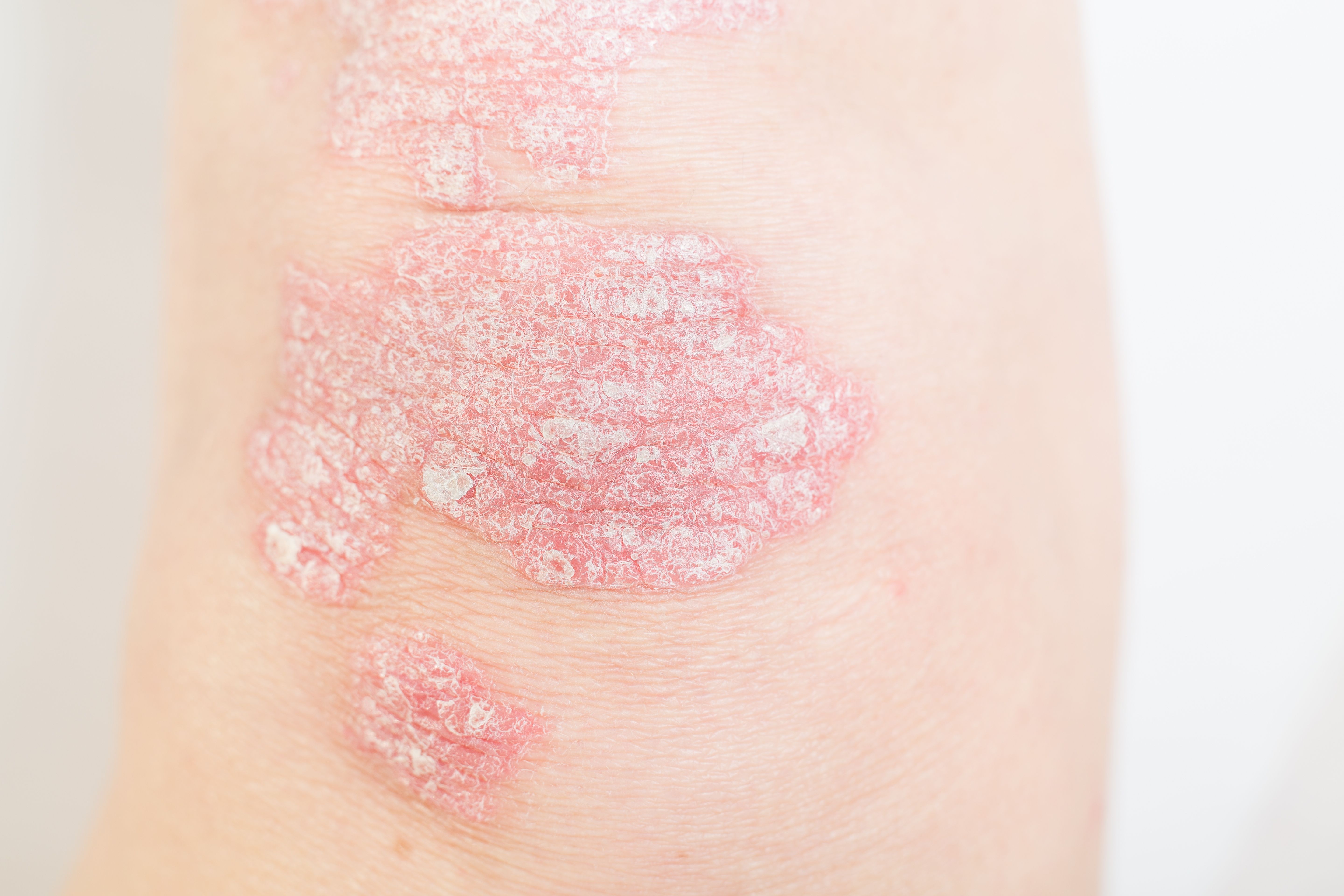
Plaque Psoriasis Symptoms Improved With Deucravacitnib Treatment
Author(s):
Investigators reported that deucravacitnib improved quality of life and clinical outcomes scores in those with moderate to severe psoriasis.
In a poster presented at Maui Derm NP+PA Summer, June 21-24, in Colorado Springs, Colorado, investigators reported that a correlation exists between clinical and patient-reported outcomes (PROs) and use of the oral, selective, allosteric tyrosine kinase 2 (TYK2) inhibitor, deucravacitnib, in those with moderate to severe psoriasis.1
The poster “Deucravacitnib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in moderate to severe plaque psoriasis: Correlations between patient-reported outcomes and clinical responses in the phase 3 clinical trials POETYK PSO-1 and POETYK PSO-2" highlighted findings in the phase 3 clinical trials POETYK PSO-1 and PSO-2, where researchers randomly assigned participants to either a placebo group, a group receiving deucravacitnib, or a group receiving apremilast.
In each clinical trial, the group receiving deucravacitnib saw a ≥75% reduction from baseline in the Psoriasis Area and Severity Index Score (PASI 75) and Static Physician’s Global Assessment scores of 0 or 1 (sPGA 0/1), and showed significant improvements on Psoriasis Symptoms and Signs Diary (PSSD) total scores (≥25 points) and Dermatology Life Quality Index (DLQI) total scores (≥4 points) when compared to participants receiving either the placebo or apremilast.
A larger number of those receiving the deucravacitnib treatment than either the placebo or apremilast reported a reduction in symptoms and improved quality of life, both in participants who did and did not achieve PASI 75 and sPGA 0/1 responses.
In POETYK PSO-1 (n=666) and PSO-2 (n=1020), adults aged 18 or older were randomized 1:2:1 to placebo, deucravacitnib 6mg once daily, or apremilast 30 mg twice daily. Participants in the studies had moderate to severe psoriasis.
At week 16, a greater number of participants receiving deucravacitnib and reached clinical response reported PSSD total score response (68.6% of those achieving PASI 75 and 68.7% of those achieving sPGA 0/1) compared with those receiving the placebo (31.3% of those achieving PASI 75 and 37.0% of those achieving sPGA 0/1).
On the PSSD itch item, 80.8% of participants who reached PASI 75 and received the deucravacitnib treatment reported meaningful improvement compared with 43.8% of participants who reached PASI 75 and received the placebo.
At week 16, across both trial groups, those treated with deucravacitnib reported a greater improvement in DLQI scores (≥4 -point reduction from baseline) than those treated with the placebo.
Investigators concluded that skin clearance, symptom reduction, and improved quality of life were correlated in the POETYK PSO-1 and PSO-2 trials. A higher clinical response was correlated with a greater PRO measure response.
Reference
- Armstrong A, Papp K, Zhuo J, et al. Deucravacitnib, an oral, selective, allosteric tyrosine kinase 2 inhibitor, in moderate to severe plaque psoriasis: Correlations between patient-reported outcomes and clinical responses in the phase 3 clinical trials POETYK PSO-1 and POETYK PSO-2. Poster presented at: Maui Derm NP+PA Summer Conference, June 21-24, 2023, Colorado Springs, Colorado.