- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Piperacillin/Tazobactam Shows Potential in Severe HS Treatment
Author(s):
Follow-up results showed that 6 out of 9 patients achieved HiSCR at 3 months, with 5 maintaining this response at 6 months.
Image Credit: © Lea - stock.adobe.com
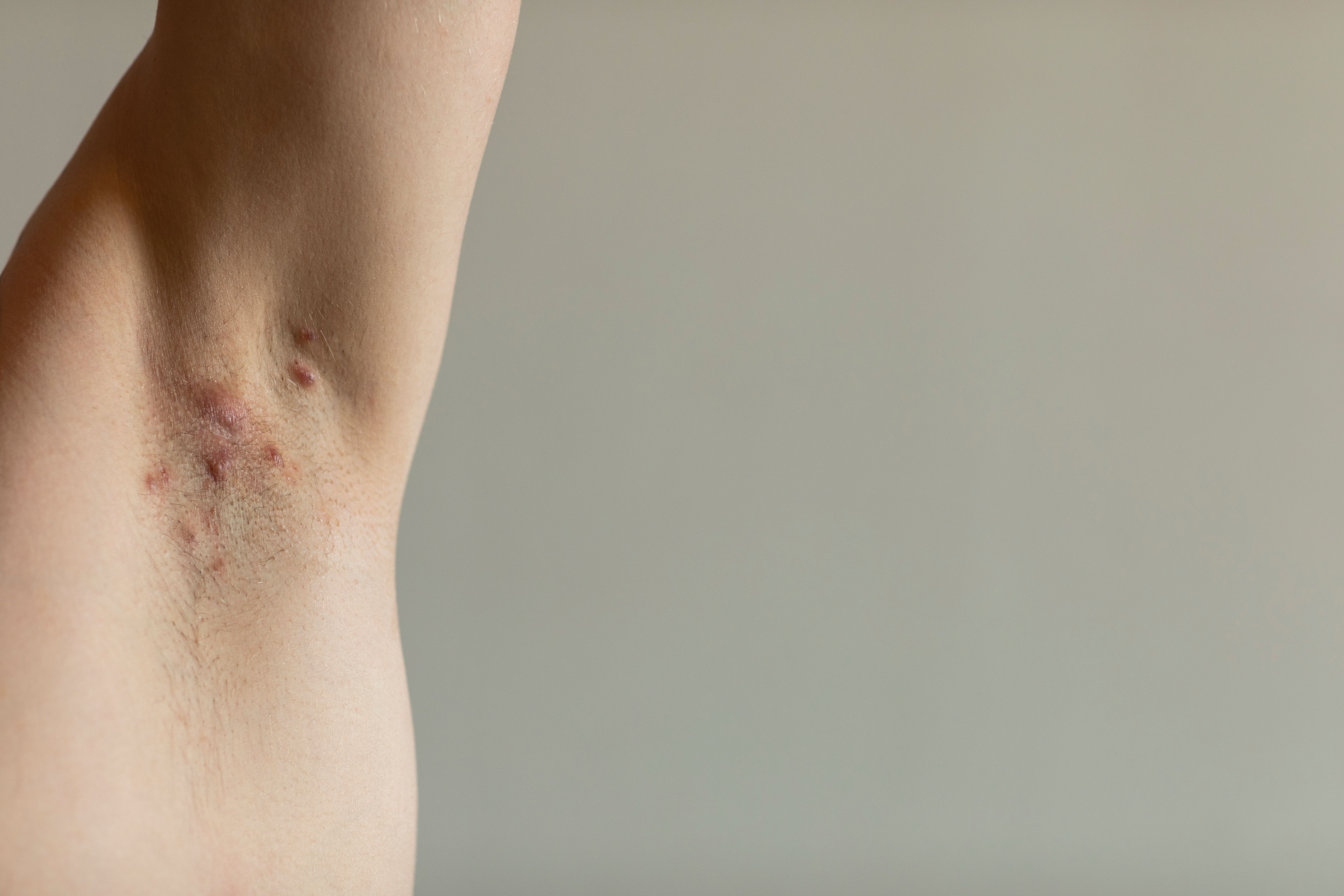
Hidradenitis suppurativa (HS)is associated with a variety of genetic, environmental, and immune factors, including changes in cutaneous microbiota.1 Although its pathophysiology is complex and not fully understood, HS has a significant impact on patients' quality of life and is linked with other pathological conditions.2
Currently, there is no definitive cure for HS, and treatment focuses on managing symptoms and preventing complications. Various methods are used to assess disease severity, including the Hurley staging system, HS-Physician Global Assessment (HS-PGA), and HS Clinical Response (HiSCR).3 Treatments include antibiotics, hormonal agents, and retinoids, with biologic therapies like adalimumab being used when other treatments fail.4 In severe cases, intravenous ertapenem or piperacillin/tazobactam may be administered. Piperacillin/tazobactam, a broad-spectrum antibiotic combination, has been used in a case series for patients with severe HS to evaluate its efficacy and safety in managing the condition, particularly focusing on its role in addressing secondary infections and influencing the associated microbiome.5-7
With this in mind, a recent study reported on a case series involving 10 patients with Hurley stage II-III HS who were treated with piperacillin/tazobactam. The treatment was started empirically to serve both as an anti-inflammatory agent and to address secondary infections and affect the HS-associated microbiome. The study hoped to assess the efficacy and safety of this approach and determine its potential benefits for managing severe HS lesions complicated by secondary infections.8
Results
The case series involved patients with Hurley stage II-III HS, including 6 women and 4 men, with a mean age of 45.2 years. The patients had severe inflammatory lesions, recurrent abscesses, and nodules affecting at least 2 anatomical regions, and their HS-PGA scores ranged from 3 to 5. Researchers stated all 10 patients were treated with intravenous piperacillin/tazobactam as an empirical therapy, with a daily dose of 13.5 g administered over an average of 9.1 days (range, 6 to 21 days). No additional therapies, including biologics, were given during this treatment period. Despite the presence of various microorganisms in lesional cultures, the study stated piperacillin/tazobactam was continued based on its empirical use. Clinical improvement was observed in 80% of patients, with “significant” reductions in inflammatory markers such as CRP and leukocyte levels.
Follow-up over 3 to 8 months showed that 6 out of 9 patients achieved HS Clinical Response (HiSCR) at 3 months, and 5 maintained HiSCR at 6 months. The study found most patients continued or adjusted biologic therapies after the initial treatment. Sustained improvement was noted in patients who adhered to treatment and had no significant comorbidities, while others experienced worsening symptoms or required additional interventions. Notably, researchers stated 1 patient with multiple health issues showed a decline in HS severity, while others faced exacerbations that necessitated changes in their treatment regimens.
Conclusion
The study found that piperacillin/tazobactam demonstrates potential as an effective third-line therapy for severe HS cases, particularly for patients with Hurley stage II and III who have not responded to other treatments. Researchers found that piperacillin/tazobactam “significantly” improved clinical symptoms and inflammatory markers, with good tolerability and notable response rates in most patients. The study noted the treatment also aligns with the need for effective interventions in managing HS flares and highlights the value of broad-spectrum antibiotics in this context. Researchers noted the study’s limitations, such as its retrospective design and small sample size, underscore the need for larger, controlled trials to validate these findings and better understand the underlying mechanisms and long-term effects of this therapy. They suggested future research should consider the implications for antibiotic resistance and the role of piperacillin/tazobactam in conjunction with biologic therapies to optimize HS management.
References
- Zouboulis CC, Benhadou F, Byrd AS, et al. What causes hidradenitis suppurativa ?-15 years after. Exp Dermatol. 2020;29(12):1154-1170. doi:10.1111/exd.14214
- Caccavale S, Tancredi V, Boccellino MP, et al. Hidradenitis suppurativa burdens on mental health: A literature review of associated psychiatric disorders and their pathogenesis. Life (Basel). 2023;13(1):189. 2023 Jan 9. doi:10.3390/life13010189
- Ingram JR, Hadjieconomou S, Piguet V. Development of core outcome sets in hidradenitis suppurativa: systematic review of outcome measure instruments to inform the process. Br J Dermatol. 2016;175(2):263-272. doi:10.1111/bjd.14475
- Alikhan A, Sayed C, Alavi A, et al. North American clinical management guidelines for hidradenitis suppurativa: A publication from the United States and Canadian Hidradenitis Suppurativa Foundations: Part II: Topical, intralesional, and systemic medical management. J Am Acad Dermatol. 2019;81(1):91-101. doi:10.1016/j.jaad.2019.02.068
- Giamarellos-Bourboulis EJ, Argyropoulou M, Kanni T, et al. Clinical efficacy of complement C5a inhibition by IFX-1 in hidradenitis suppurativa: an open-label single-arm trial in patients not eligible for adalimumab. Br J Dermatol. 2020;183(1):176-178. doi:10.1111/bjd.18877
- Keikha M, Soori T, Azadi D, et al. The first report of Pseudomonas oryzihabitansin infection in a patient with hidradenitis suppurativa. Clin Case Rep. 2019;7(8):1514-1517. 2019 Jun 25. doi:10.1002/ccr3.2265
- Parrado R, Cadena M, Vergara A, et al. The role of negative pressure wound therapy in the management of hidradenitis suppurativa: a case report and literature review. Int Wound J. 2017;14(1):35-39. doi:10.1111/iwj.12544
- Barzilai A, Toubiana S, Dalal A, Baum S. The role of piperacillin/tazobactam in the treatment of Hidradenitis suppurativa. J Dermatolog Treat. 2024;35(1):2363318. doi:10.1080/09546634.2024.2363318




