- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Photodynamic therapy for nonmelanoma skin cancers: Nonsurgical route is safe, effective
Photodynamic therapy is a safe, effective treatment for nonmelanoma skin cancers, according to an expert. Though the therapy is not yet approved for the treatment of skin cancer in the U.S., It has been accepted, approved, and recommended as a potential first-line of therapy for superficial basal cell carcinomas, and some superficial squamous cell carcinomas outside the U.S.

Key Points

Photodynamic therapy is used safely and effectively to treat nonmelanoma skin cancers outside of the United States; however, the therapy is not yet approved here.
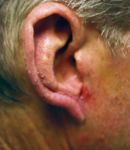
Nonmelanoma skin cancers
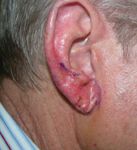
"A surgical procedure, such as conventional excision, electrodesiccation and curettage (ED&C), or an elliptical incision would be used in the U.S. If the nonmelanoma skin cancer is on the face or other sensitive area, Mohs micrographic surgery would be used," Dr. Gold tells Dermatology Times.
The treatment uses the drug Metvix (methyl aminolevulinate, Photocure ASA, Galderma Worldwide).
According to Dr. Gold, for photodynamic therapy of nonmelanoma skin cancers Metvix is placed on the lesion, which is then covered thefor three hours and then treated with a red-light laser.
"Skin cancers such as this are treated twice over two weeks," he says. "The cure rates are comparable to most surgical procedures, and they have five-year studies that support this treatment."
Multiple uses
Another photodynamic therapy, Levulan (aminolevulinic acid HCl, DUSA Pharmaceuticals), is approved for the treatment of actinic keratosis (AK) in the United States. Levulan has been approved since 1999 for actinic keratosis treatment using a blue-light laser.
"Some dermatologists, such as myself, will use blue-light or pulse-diode lasers. This treatment gives wonderful efficacy; it is safe and it is a short-contact, full-face therapy. We have lots of happy patients."
Metvix will be known as Metvixia for marketing in the United States. The drug is currently approved for the treatment of pre-cancerous lesions or actinic keratosis using a red light, but it is not on market at this time.
"Until Galderma makes a corporate decision to go after the actinic keratosis market, there is only one drug in the U.S., which is Levulan," Dr. Gold says.
"Whether or not they are going to end up with a nonmelanoma skin cancer indication for Metvixia is yet to be determined. The company is in talks with the FDA (Food and Drug Administration)," he says.
DUSA, maker of Levulan, is reportedly not pursuing a nomelanoma skin cancer indication at this time. However, Dr. Gold says, anecdotally, several small studies in the United States have looked at giving a repeated course of PDT with Levulan to those patients who are immunocompromised.
For example, it is known that kidney transplant patients develop more actinic keratosis and squamous cell carcinomas than the average person. With repeated doses of PDT with Levulan, they have shown over a year that the number of presumed nonmelanoma skin cancers is significantly reduced. Therefore, there is anecdotal evidence of a chemopreventative role with this treatment.
"Many of us in our own clinical practices have seen this," he says.
In Europe, similar studies have been conducted with PDT and Metvix for immunocompromised patients and have resulted in similar outcomes.





