- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Phase 3 results show abrocitinib safe, effective for atopic dermatitis
Author(s):
Pfizer announces top-line results from its third phase 3 clinical trial investigating abrocitinib in adults with moderate-to-severe atopic dermatitis.
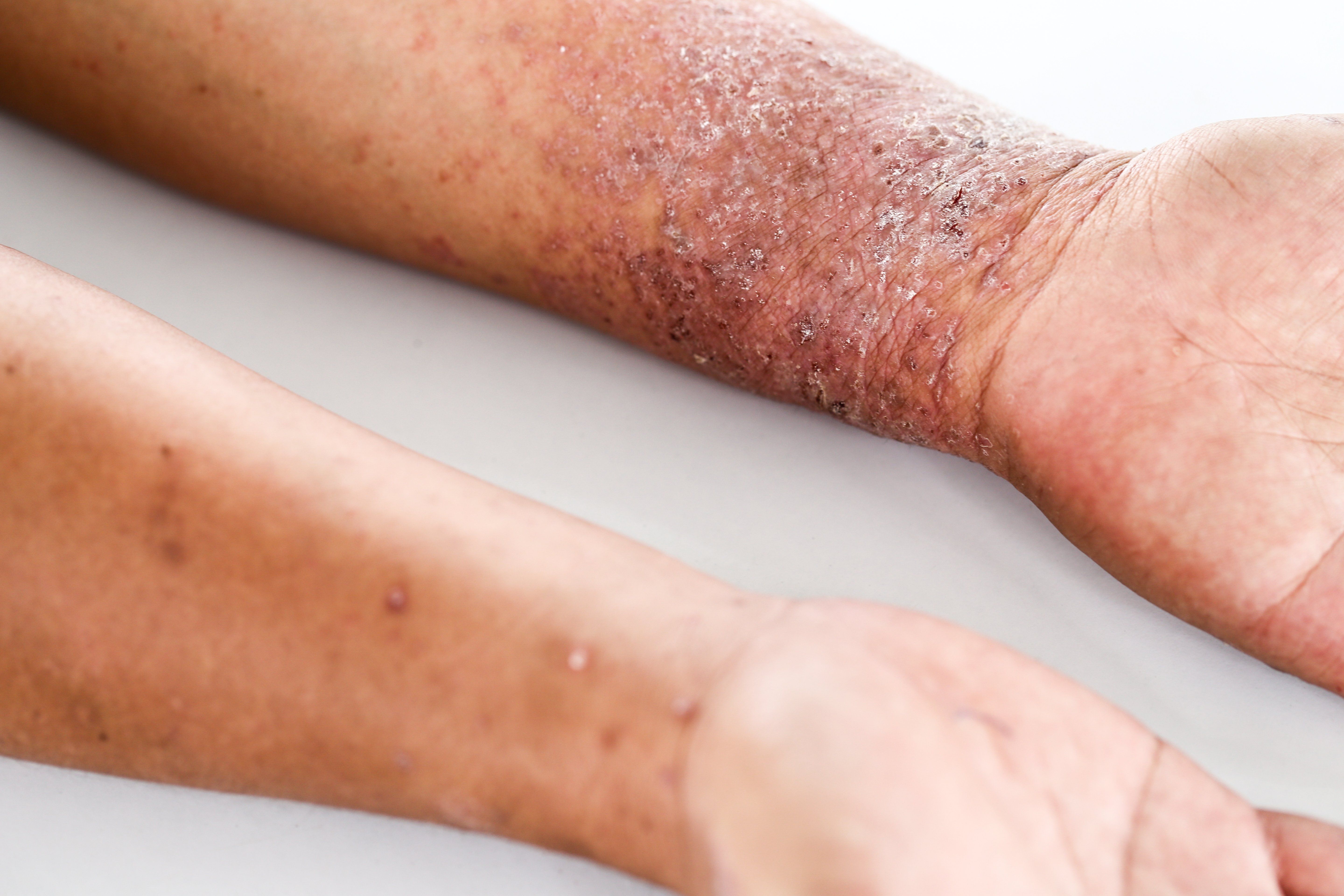
Recently published results from a phase 3 comparative study investigating abrocitinib (PF-04965842, Pfizer) indicate that the once daily oral Janus kinases 1 (JAK1) inhibitor may be an effective and safe treatment for adults with moderate-to-severe atopic dermatitis (AD). The drug received Breakthrough Therapy Designation from the U.S. Food and Drug Administration in February 2018.1
MORE: Atopic dermatitis pipeline full of potential
JAK1 inhibition is said to influence interleukin (IL)-4, IL-13, IL-22, IL-31and interferon gamma – all of which are thought to be involved in the pathophysiology of atopic dermatitis. The phase 3, double-blind, double-dummy, parallel group, placebo-controlled, multi-center clinical trial compared the safety and efficacy of abrocitinib and dupilumab verses placebo in adults with moderate-to-severe atopic dermatitis who were also receiving topical therapy.2
This comparative study (JADE COMPARE) is the third trial in the JAK1 Atopic Dermatitis Efficacy and Safety (JADE) global development program, which has previously released positive results from companion studies investigating the use of abrocitinib as a monotherapy (JADE MONO-1 and JADE MONO-2).
The 837 participants were randomized to five groups. Two treatment arms received once-daily oral doses of abrocitinib 100 mg or 200 mg along with a subcutaneous injection of placebo for 16 weeks, then given the same dose of abrocitinib once a day during weeks 16-20, respectively. In one of the three active comparator arms, subjects were subcutaneously injected with dupilumab (Dupixent, Sanofi and Regeneron Pharmaceuticals) 300 mg (600 mg loading dose at baseline) once every two weeks and took an oral placebo for 16 weeks, subsequently followed-up by taking the oral placebo from weeks 16-20. The other two comparator arms received oral and injectable placebos for 16 weeks and then followed by 100 mg or 200 mg doses of abrocitinib once daily from weeks 16-20.3
"It was helpful to study abrocitinib in combination with topical therapies to provide data relevant to the real-world setting," says Michael Corbo, PhD, chief development officer for inflammation and immunology at Pfizer. "The addition of an active control was also important to better understand the significance of this potential new medicine and we're encouraged by the positive data from this trial."
Co-primary endpoints of the study include a proportion of subjects who achieved an Investigator’s Global Assessment (IGA) score of clear (0) or almost clear (1) and a two-point or more reduction from the baseline at week 12 as well as a proportion of participants who achieved 75% or more change from baseline at week 12 in their Eczema Area and Severity Index (EASI) score.
Additionally, secondary endpoints include the proportion of subjects achieving a four-point or more reduction in itch severity from baseline at week 2 according to the Peak Pruritis Numerical Rating Scale (PP-NRS), and the proportion of patients at week 16 achieving the EASI and IGA measures.
Results of the trial show all co-primary endpoints were met and both doses of abrocitinib were displayed superiority to placebo at week 16 and was sustained until week 16. Dupilumab also exhibited superiority over placebo during both week 12 and 16.
RELATED: Atopic dermatitis patients need better disease control
Additionally, the safety profile was comparable to previous JADE studies. Results reveal 61.9% of subjects who received abrocitinib 200 mg experienced adverse events, whereas adverse event rates for dupilumab, placebo and abrocitinib 100 mg were 50%, 53.4% and 50.8%, respectively.
Pfizer plans to use the data from the JADE trials to support filings with the FDA further down the road in 2020 and full study results will be published in an upcoming scientific journal.
References:
1. Pfizer Announces Positive Top-Line Results from Third Phase 3 Trial of Abrocitinib for Moderate to Severe Atopic Dermatitis, Which Showed Improvements in Skin Clearance, Disease Extent and Severity, and Itch. Pfizer. https://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_positive_top_line_results_from_third_phase_3_trial_of_abrocitinib_for_moderate_to_severe_atopic_dermatitis_which_showed_improvements_in_skin_clearance_disease_extent_and_severity_and_itch. Published March 18, 2020. Accessed March 23, 2020.
2. Study Evaluating Efficacy and Safety of PF-04965842 and Dupilumab in Adult Subjects With Moderate to Severe Atopic Dermatitis on Background Topical Therapy - Full Text View. Study Evaluating Efficacy and Safety of PF-04965842 and Dupilumab in Adult Subjects With Moderate to Severe Atopic Dermatitis on Background Topical Therapy - Full Text View - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT03720470. Published March 23, 2020. Accessed March 25, 2020.
3. Pfizer reports positive results from third Phase III study of JAK1 inhibitor abrocitinib in atopic dermatitis. FirstWord Pharma. https://www.firstwordpharma.com/node/1709141?al=3a2380-a882871cf1564cc5f229595ac3bb61d7^|^MTEwOTcyOA==^|^NQ==&cp1=bmV3c2xldHRlcl9yZWdpb25faWQ9dG9wX25ld3M. Published March 18, 2020. Accessed March 20, 2020.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.






