- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Phase 2 Study of Ruxolitinib Cream for Vitiligo Announces New Findings
Author(s):
Incyte's Phase 2 study evaluating ruxolitinib cream for vitiligo meets primary endpoint, shows improvement in body and facial repigmentation compared to placebo.
Incyte recently announced findings from 2 analyses of its randomized, dose-ranging, vehicle-controlled Phase 2 study evaluating ruxolitinib cream, an investigational nonsteroidal, anti-inflammatory, JAK inhibitor therapy, in adult patients with vitiligo. These presentations (Poster #27535 and #27568) will be available on-demand as part of the American Academy of Dermatology Virtual Meeting Experience 2021 (AAD VMX), held virtually from April 23–25, 2021.
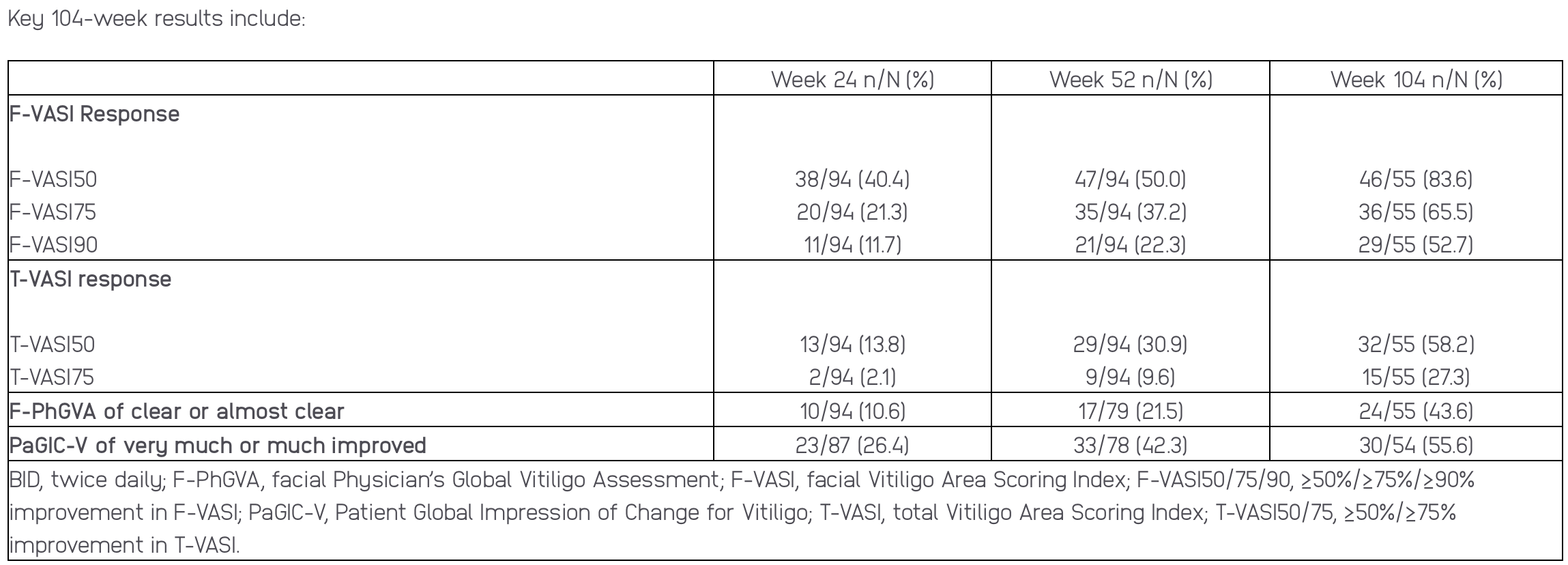
As previously announced, the Phase 2 study met its primary endpoint, demonstrating that significantly more patients treated with ruxolitinib cream for 24 weeks achieved a ≥50% improvement from baseline in the facial vitiligo area scoring index (F-VASI50) score compared to patients treated with a vehicle control (non-medicated cream). Fifty-two-week results were also previously reported.
Updated results at Week 104 (Poster #27535) continue to show improvements in facial repigmentation, measured by F-VASI50 score, and total body repigmentation, measured by the proportion of patients achieving a ≥50% improvement from baseline in the total vitiligo area scoring index (T-VASI50), a key secondary endpoint, with 1.5% ruxolitinib cream administered twice daily. The results also add to previous findings indicating that a longer duration of therapy was associated with greater repigmentation, as assessed using the F-VASI and T-VASI responses at Weeks 24, 52 and 104.
Table courtesy of Incyte press release.
“Vitiligo is a chronic autoimmune disease associated with significant quality of life impairments that currently has no FDA approved treatments available,” said Jim Lee, MD, PhD, group vice president, Inflammation & Autoimmunity, Incyte. “These findings continue to point to the potential of ruxolitinib cream to provide meaningful facial and total body repigmentation that continues to improve over time for patients who seek treatment for vitiligo. We look forward to further evaluating ruxolitinib cream in this patient population and to sharing results of our Phase 3 TRuE-V studies later this year.”
Separately, an exploratory analysis evaluated the maintenance of repigmentation among patients in the Phase 2 study who responded to ruxolitinib cream and then discontinued treatment after 104 weeks (Poster #27568). The findings suggest that the repigmentation seen with ruxolitinib cream may be maintained after treatment discontinuation.
Specifically:
- 12 of 16 patients (75.0%) maintained total body repigmentation and 13 patients (81.3%) maintained facial repigmentation during follow-up duration of 1 to 6 months.
- No patients initially randomized to 1.5% ruxolitinib cream twice daily (n=3; with 2 years’ exposure) experienced loss of repigmentation at Week 104 vs previous follow-up.
Across both analyses, the overall safety profile of ruxolitinib cream was consistent with previously reported data, and no new safety signals were observed.
“As a physician who treats serious dermatologic conditions like vitiligo, I aim to offer my patients treatments that may help them achieve their goals,” said David Rosmarin, MD, vice chair of research and education, Dermatology Department at Tufts Medical Center. “I am encouraged by the ruxolitinib cream vitiligo data presented at AAD VMX, including the continued improvement in outcomes and duration of results that were observed in the analyses, and look forward to seeing more from the ongoing evaluation of this potential topical treatment in patients with vitiligo who currently have limited options for skin repigmentation.”
These presentations are available on-demand on the AAD VMX website and can be accessed until July 12, 2021.
Reference:
Incyte Announces New Findings from a Randomized Phase 2 Study of Ruxolitinib Cream in Patients with Vitiligo. Incyte. April 23, 2021. Accessed April 24, 2021. https://investor.incyte.com/press-releases/press-releases/2021/Incyte-Announces-New-Findings-from-a-Randomized-Phase-2-Study-of-Ruxolitinib-Cream-in-Patients-with-Vitiligo/default.aspx






