- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Phase 1b Study of Rezpegaldesleukin in Moderate-to-Severe AD Met Clinical Efficacy Endpoints
Author(s):
Nektar Therapeutics announced the promising and statistically significant results, which included meeting endpoints of BSA, DLQI and POEM in patients with atopic dermatitis.
Nektar Therapeutics has announced1 positive and promising new data stemming from its phase 1b study of rezpegaldesleukin in patients with moderate to severe atopic dermatitis (AD). The study met several clinical efficacy endpoints with statistically significance, including Body Surface Area (BSA), Dermatology Life Quality Index (DLQI), and Patient-Oriented Eczema Measure (POEM).
lial88/Adobe Stock
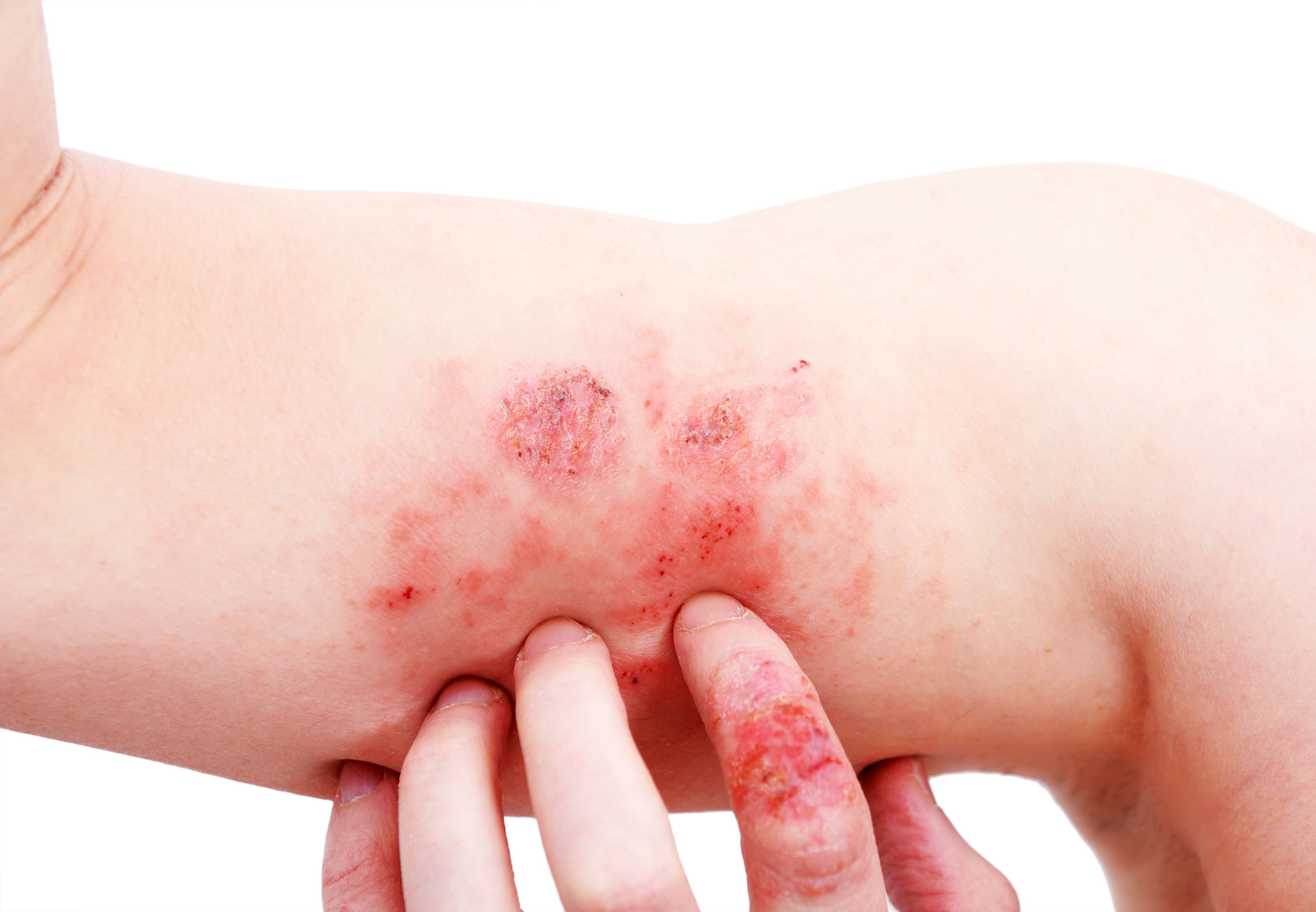
The study, which was double-blind, placebo-controlled, and randomized, evaluated the safety, tolerability, and pharmacokinetics of the first-in-class selective regulatory T-cell therapeutic. Patients with more severe AD were evaluated over a 12-week induction treatment period and assigned to 1 of 3 treatment arms, including 12 µg/kg rezpegaldesleukin, 24 µg/kg rezpegaldesleukin, and a placebo.
From baseline to the conclusion of the 12 week period, patients receiving either dose of rezpegaldesleukin had significant improvements in their average Eczema Area and Severity Index (EASI) scores, with an average 65% improvement in patients receiving 12 µg/kg and 83% improvement in patients receiving 24 µg/kg. This is compared to 47% improvement in patients assigned to the placebo.
Furthermore, as observed, 29% of patients receiving the placebo achieved EASI-75 at 12 weeks, compared to 33% in the 12 µg/kg group and 58% in the 24 µg/kg group. These improvements were also evident when calculated using non-responder imputation.
Additionally, patients receiving either dose of rezpegaldesleukin achieved statistically significant improvements in average percentage of BSA, DLQI, and POEM scores.
"These new data announced today for rezpegaldesleukin in atopic dermatitis demonstrate that, in addition to the strong previously reported efficacy for EASI-related endpoints, rezpegaldesleukin has the potential to be a differentiated therapeutic that could greatly improve quality-of-life for patients," said Jonathan Zalevsky, PhD, chief R&D officer of Nektar Therapeutics, in a press release.1
Research into the efficacy of rezpegaldesleukin is ongoing, according to Nektar Therapeutics, who anticipates initiating a phase 2b study of the drug in biologic-naïve patients with moderate to severe AD this October. A phase 2a study of rezpegaldesleukin in patients with alopecia areata is expected to begin in early 2024.
Reference
- Therapeutics N. Nektar Therapeutics announces promising new data from Phase 1B study of Rezpegaldesleukin in moderate-to-severe atopic dermatitis. PR Newswire: press release distribution, targeting, monitoring and marketing. September 13, 2023. Accessed September 13, 2023. https://www.prnewswire.com/news-releases/nektar-therapeutics-announces-promising-new-data-from-phase-1b-study-of-rezpegaldesleukin-in-moderate-to-severe-atopic-dermatitis-301925787.html.






