- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
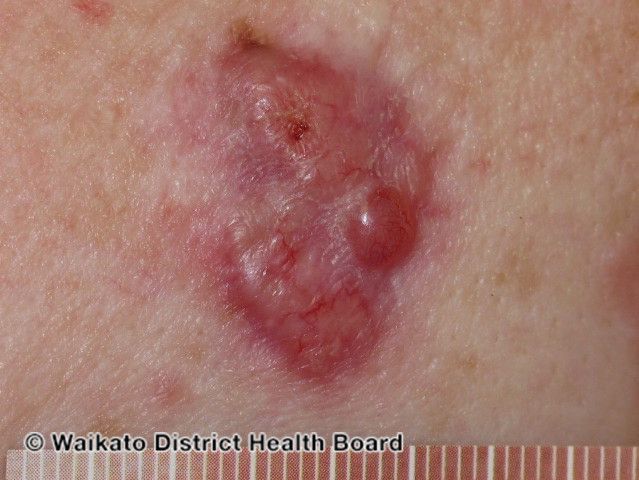
Phase 1/2 Trial of AIV001 (Axitinib) for Basal Cell Carcinoma Concludes With Promising Data
Author(s):
The study evaluated the efficacy of AIV001 in superficial, nodular, and mixed BCC tumors.
AiViva Biopharma announced today positive and promising data from its phase 1/2 clinical trial of AIV001 (Axitinib), a novel intradermal treatment, in patients with basal cell carcinoma (BCC).
“We are excited to have completed this safety study that is demonstrating excellent skin tolerability and a dose response," said Diane Tang-Liu, PhD, President and CEO of AiViva, in a press release. "This promising data allows AiViva multiple options to develop it further, including but not limited to seeking a strategic partner for this compound."
The multicenter, open-label trial evaluated AIV001 in nodular, superficial, and mixed nonmelanoma BCC tumors. Twenty-six patients with BCC tumors were evaluated within the study.
AIV001 was administered in 4 ascending dose cohorts with up to 3 treatments administered to each patient throughout the duration of the study, as determined necessary on a case-by-case basis.
Investigators evaluated patients for safety, efficacy, and skin tolerability.
“It is exciting and important to have AiViva Biopharma develop a drug, with a new mechanism of action, that has the potential to treat BCC that avoids surgery,” said Brian Berman, MD, PhD, in a press release. Berman is Professor Emeritus of Dermatology and Dermatologic Surgery at The University of Miami Miller School of Medicine.
Currently, AIV001 is in development for nonmelanoma skin cancer, BCC, papulopustular rosacea, and the treatment of scars and wounds.
Reference
BioSpace. AiViva biopharma completes phase 1/2 clinical trial of AIV001 administration in patients with nonmelanoma skin cancer. BioSpace. December 11, 2023. Accessed December 13, 2023. https://www.biospace.com/article/releases/aiviva-biopharma-completes-phase-1-2-clinical-trial-of-aiv001-administration-in-patients-with-nonmelanoma-skin-cancer/#:~:text=COSA%20MESA%2C%20Calif.%2C%20Dec,cell%20carcinoma%20(BCC)%20tumors