- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
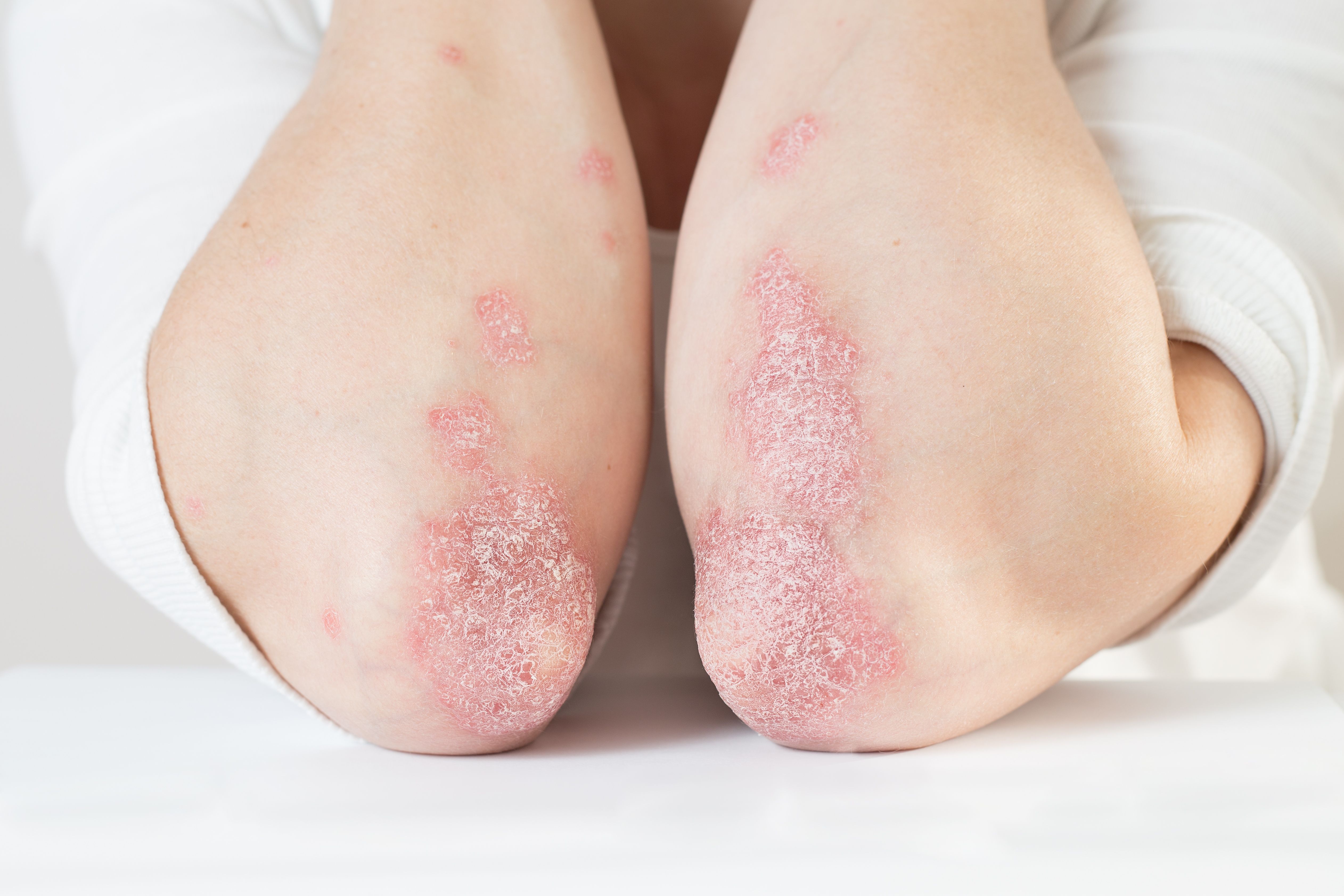
Oral apremilast effectively treats scalp psoriasis
Author(s):
A recent article shows oral apremilast may rapidly and significantly improve moderate-to-severe scalp psoriasis.
Oral apremilast (Otezla, Amgen) rapidly and significantly improved disease severity, itch and quality of life compared with placebo in patients with moderate-to-severe scalp psoriasis, according to an article in press in the Journal of the American Academy of Dermatology (JAAD).1
RELATED: Phase 3 results show apremilast safe, effective for plaque psoriasis
Eight in 10 psoriasis patients have scalp involvement, making the scalp the most commonly affected area in psoriasis. People who have scalp psoriasis often suffer significantly when it comes to their mental health, quality of life and social functioning. Despite the condition’s impact, patient adherence to first-line topical therapies tends to be low.
“There is a need for effective, well-tolerated systemic therapies for patients with moderate to severe scalp psoriasis or patients whose scalp psoriasis is inadequately controlled with topical therapies,” the authors write.
In this phase 3b, double-blind, placebo-controlled study, researchers randomized 201 patients with moderate-to-severe scalp psoriasis to receive 30 mg twice daily of apremilast, a phosphodiesterase 4 inhibitor, or placebo for 16 weeks. The patients at 41 sites in the United States and Canada were 18 years and older, had Scalp Physician Global Assessment scores of three or more, 20% or more of scalp surface area involvement and had not tolerated or had inadequate response to one or more topical therapies for scalp plaque psoriasis.
They found that 43.3% of patients taking apremilast achieved the primary endpoint of clear or almost clear with a two or greater point reduction on the Scalp Physician Global Assessment. That was compared to 13.7% in the placebo group.
More than 45% of patients in the apremilast arm achieved a four-point or greater improvement from baseline according to the Whole Body Itch Numeric Rating Scale, compared to 22.5% of placebo. And 47.1% in the apremilast group achieved a four-point or greater improvement according to the Scalp Itch Numeric Rating Scale, versus 21.1% in the placebo group.
Patients taking apremilast also had a significantly greater average improvement in the Dermatology Life Quality Index than those in the placebo arm.
RELATED: Apremilast appears safe for children with psoriasis
More than 30% of patients on apremilast had diarrhea. Other common adverse events in the apremilast group were nausea, headache and vomiting.
This is the first prospective, randomized, placebo-controlled phase 3 study looking at apremilast treatment for moderate-to-severe scalp psoriasis, the authors note.
“Scalp psoriasis can be a very distressing manifestation of psoriasis, as it is in a highly visible location and often causes intense pruritus,” they write. “The clinically meaningful improvements in Scalp and Whole Body Itch [Numeric Rating Scale] are supported by the significantly greater improvements in quality of life among patients treated with apremilast versus placebo.”
Psoriasis location is one of many factors that providers must consider when prescribing treatment. Results from this study might not be generalizable to patients with other psoriasis forms, according to the paper.
As the analysis did not provide long-term efficacy data and the study did not compare apremilast to other potential treatments for scalp psoriasis, the authors note these as limitations.
Disclosures:
Celgene Corporation, which sold Otezla to Amgen in 2019, funded the study and the authors received editorial support from a company sponsored by the Celgene Corporation. The authors of the study have ties to Celgene and other corporations, including as researchers, consultants and advisory board members.
Reference:
Van voorhees AS, Stein gold L, Lebwohl M, et al. Efficacy and safety of apremilast in patients with moderate to severe plaque psoriasis of the scalp: Results of a phase 3b, multicenter, randomized, placebo-controlled, double-blind study. J Am Acad Dermatol. 2020;83(1):96-103.