- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
One test helps inform two key clinical decisions in the management of melanoma
Sponsored By:

Melanoma is one of the most dangerous skin cancers and carries significant morbidity and mortality for patients, thus early diagnosis with appropriate risk-aligned management is key to ensure the best possible outcome for each patient. After a melanoma diagnosis is made, clinicians are confronted with multiple critical decisions about the management of the patient’s melanoma. Patients for whom definitive surgical excision is feasible are routed for excision with appropriate margin control, and additional risk-aligned management decisions must be made by the treating clinician/dermatologist, such as referral for sentinel lymph node (SLN) biopsy, setting the frequency of clinical follow-up, referral for initial advanced/surveillance imaging and referral to other specialists (e.g., medical oncologists), all of which are dependent on the patient’s risk of recurrence and metastasis. Recommendations for patient care outlined by the consensus-driven National Comprehensive Cancer Network (NCCN) are based on cancer stage, as defined by the American Joint Committee on Cancer 8th edition (AJCC8) staging criteria.1,2AJCC8 considers various clinical and pathological features of a patient’s primary melanoma tumor to classify the tumor’s stage and provide population-based risk estimates to help guide these critical patient management decisions.
Figure 1: AJCC8 staging criteria and NCCN/SSO guidelines for SLN biopsy decision-making
T-Stage is an important part of the patient selection process for SLN biopsy surgery and is based on Breslow thickness and ulceration status.†
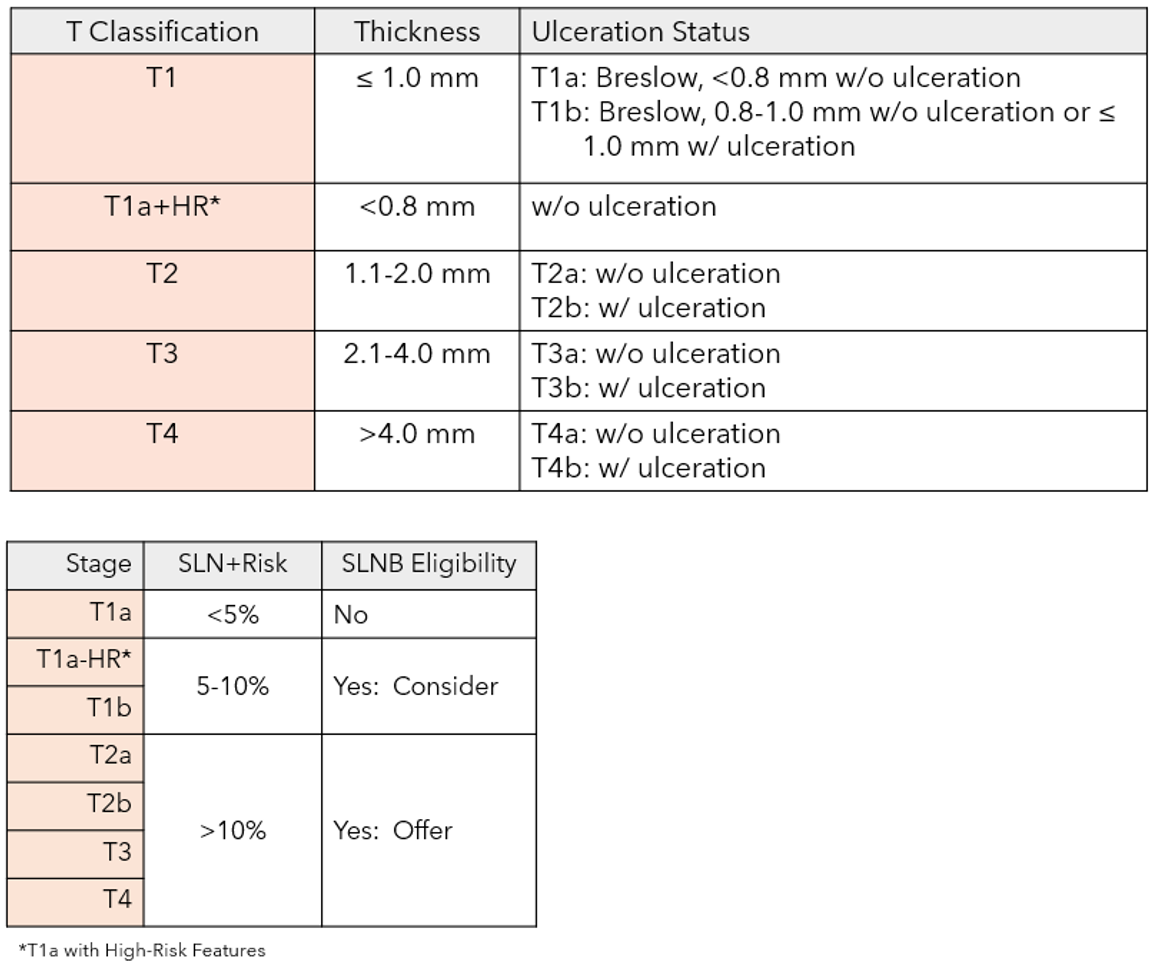
†NCCN Guidelines for Melanoma v1.2021, ASCO/SSO Guidelines for Sentinel Lymph Node Biopsy 2017, AAD Guidelines for Melanoma 2018; *Other high-risk (HR) factors include very high mitotic rate or mitotic rate ≥2mm2, lymphovascular invasion, uncertain microstaging and young age.
Risk stratification approaches that rely only on clinicopathologic factors may under- or overestimate the risk of a patient with melanoma
Currently, AJCC8 melanoma staging is determined by only three clinicopathological factors strongly correlated with cancer severity: Breslow depth (tumor thickness), ulceration in the primary tumor and evidence of tumor-spread to the SLN, in-transit disease or distant metastasis. As AJCC8 includes only this limited set of factors, clinicians may take into account other risk factors, such as comorbidities and family history, and use information that is standardly reported with a melanoma diagnosis (e.g., melanoma subtype, lymphovascular invasion, mitotic rate, patient age, etc.) to make more informed management decisions about a patient’s care.3
While AJCC8 is valuable and does risk stratify patients, it relies on population-based risk estimates, which fail to take into account a patient’s unique tumor biology. These population-based estimates may overestimate the metastatic risk of an individual’s disease, or worse, underestimate it, missing patients with aggressive tumor biology. Melanoma staging based on clinicopathologic factors alone does not provide complete certainty about a patient’s individual risk of recurrence or disease-specific death, particularly for those with early stage I or II tumors.5-7 These accuracy limitations are evidenced by the fact that, when removing stage IV, more than half of the deaths caused by melanoma occur in patients who were originally diagnosed with stage I or II tumors, and thus are presumed to have a low risk of recurrence from a population-based perspective. Yet many tumors diagnosed as stage I or II eventually recur after excision.4-6Tests that help refine prognostic accuracy, or that can be used in combination with current staging, have the potential to improve risk-aligned management decisions for the benefit of patient care.
Figure 2: melanoma staging and mortality

References: AJCCv7 J Clin Oncol 2009; SEER program database (released April 2022); Whiteman D et al. J Invest Derm 2015; Shaikh W et al. J Natl Cancer Inst 2016
The 31-GEP (DecisionDx-Melanoma; Castle Biosciences, Inc., Friendswood, TX) is a molecular gene expression profile (GEP) test that provides tumor-specific information for improved treatment decisions and patient outcomes. The test has been commercially available since May 2013 and provides personalized results that can help improve patient risk stratification and guide risk-aligned management decisions. The 31-GEP test is covered by Medicare, as well as multiple private insurers, and has seen widespread adoption in the nine years that it has been clinically available, with more than 100,000 clinical test orders from more than 10,200 providers. While national recommendations for risk assessment have not yet included tumor biology, centers utilizing the test are generating impactful results that drive center-specific patient workflows that incorporate testing with the 31-GEP.7-9 And similarly, clinicians who have seen the potential benefits of using the 31-GEP test with their own patients continue to utilize the test to guide more informed, risk-based treatment plans and potentially better outcomes, making personalized medicine a reality for patients with melanoma.
Genomic testing to optimize the management of invasive cutaneous melanoma (CM)
Understanding each patient’s individual risk level is critical to improving outcomes for patients diagnosed with melanoma. Moreover, a clinician’s ability to make accurate prognoses can be enhanced by gathering input from all available features that carry high predictive value, including the molecular biology of a patient’s tumor, or more specifically, its gene expression signature. Through the use of GEP tests in melanoma prognoses, clinicians can gain more precise and personalized information about a patient’s risk of recurrence and metastasis, beyond what is possible using traditional clinicopathologic staging alone. The use of gene expression profiling was most notably implemented in the field of breast cancer two decades ago, where the 21-GEP test, OncotypeDX® (Genomic Health), was applied clinically to identify the likelihood of response to chemotherapy.
Numerous clinical studies have supported the use of GEP testing in melanoma to improve the risk stratification of patients and guide more informed and personalized management decisions better aligned with each patient’s biological risk of metastasis and recurrence. Backed by more than 35 peer-reviewed, published studies including more than 9,000 patient samples, the 31-GEP test measures the expression of 31 genes, using tissue from the initial tumor biopsy, to predict the likelihood that a patient’s cancer will metastasize or recur.10 The test classifies a patient’s tumor as being at lowest risk (Class 1A), increased risk (Class 1B/2A) or highest risk (Class 2B) of recurrence or metastasis within five years and is indicated for patients with stage I-III invasive cutaneous melanoma when the test’s result will impact important patient management decisions.
Genetic classifiers that contain clinicopathologic factors must first demonstrate independence. This level of statistical independence – that the genetic classifier provides information independent from, and therefore not obtainable from, known clinicopathologic factors – is an important step in developing a useful classifier, and only the 31-GEP test has demonstrated this evidence. The risk-stratification provided by 31-GEP test results is independent of the most important clinicopathologic risk factors traditionally used in melanoma risk assessments (e.g., Breslow thickness, ulceration, age and SLN biopsy status), with an accuracy that can improve upon current AJCC8 staging alone.11,12 In fact, when comparing the test’s risk stratification capabilities to the most relevant clinicopathologic risk factors in multivariate models, the 31-GEP test has been shown to be a consistent and independent predictor of patient outcomes (melanoma-specific survival (MSS), distant metastasis-free survival (DMFS) and recurrence free survival (RFS)).13 Consistent and demonstrated analytical validity, clinical validity and clinical utility were cited in the local coverage determination (LCD) that determined the 31-GEP test would be covered by Medicare.13
Two critical treatment decisions: one test
The 31-GEP test uses powerful artificial intelligence that combines a patient’s gene expression profile information with his/her clinical and pathologic risk factors to provide more precise, personalized risk predictions and actionable results to guide risk-aligned patient care.
The 31-GEP is the only commercially-available GEP test for melanoma prognosis that has been validated, both independently and combined with clinicopathologic factors, to inform two critical questions that are key in determining the appropriate treatment and management of a patient’s melanoma:
- What is the patient’s risk of a positive sentinel lymph node (SLN)?14 and
- What is the patient’s individual risk of melanoma recurrence?15
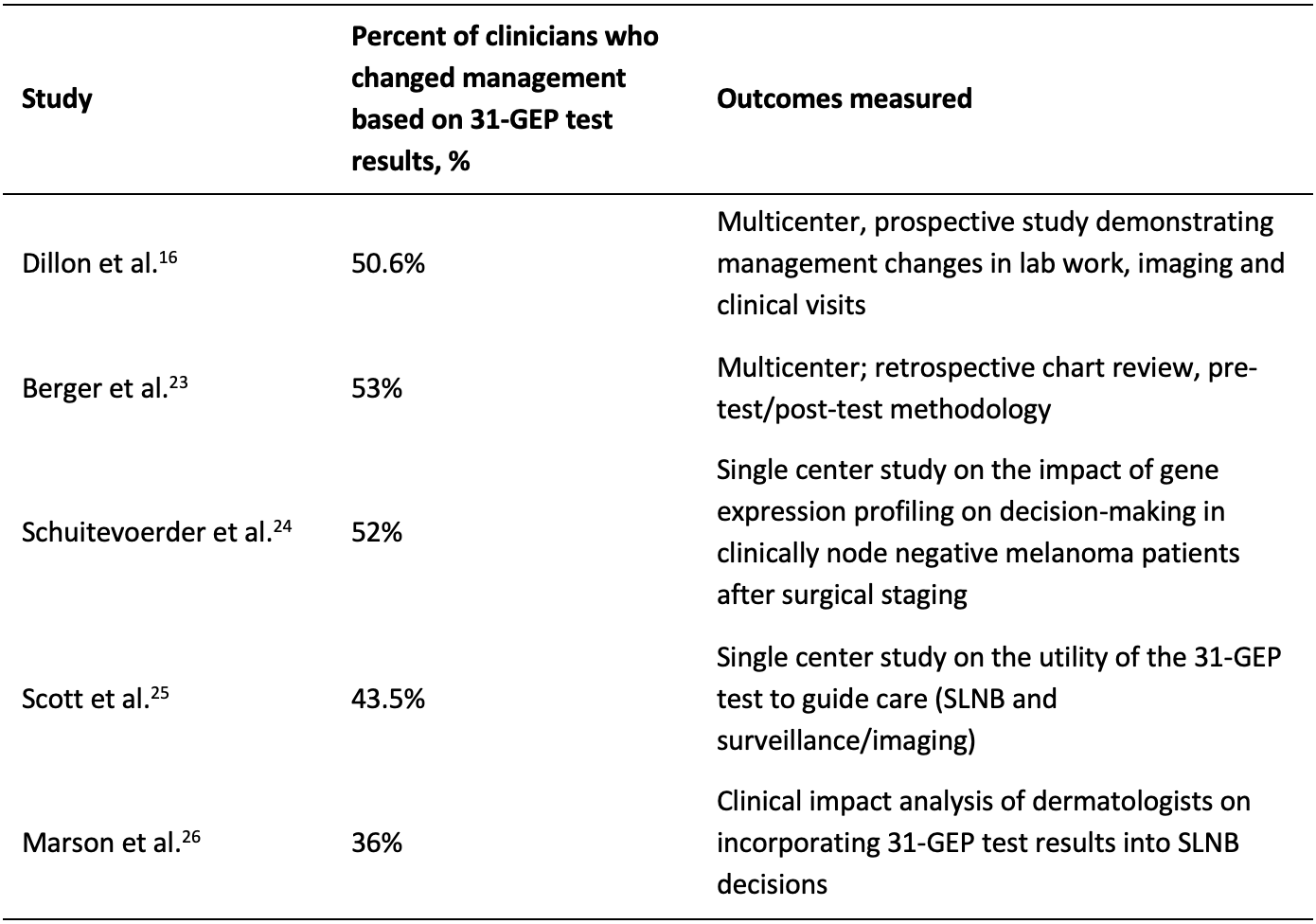
Because the 31-GEP can serve as an independent predictor of risk by measuring the expression of 31 genes and accounting for the molecular biology of a patient’s tumor, clinicians can supplement their patient care plans by considering the test’s results alongside their existing risk-assessment frameworks provided by AJCC8 staging.16 When a patient’s individualized genomic information is considered alongside traditional clinicopathologic risk factors, physicians and patients can make more informed disease management decisions better aligned to each patient’s unique biological risk. In clinical utility studies, the 31-GEP test has been shown to prompt clinicians to change their management of patients after receiving the test’s results.16, 23-26
Figure 3: changes in patient management with 31-GEP test results
Personalized Risk of SLN Positivity
The 31-GEP test result can be used in conjunction with current AJCC8 staging information to inform treatment decisions and provide a precise and personalized prediction of risk of SLN positivity. Additionally, the 31-GEP test has been shown to add positive net benefit and clinical utility for SLN biopsy decision making in patients with thin-to-intermediate thickness invasive CM.14, 17-18
Personalized Risk of Melanoma Recurrence
To help guide decisions regarding patient follow-up and treatment intensity, the test provides personalized predictions of patient-specific outcomes, including five-year MSS, DMFS and RFS. These three end points are particularly valuable as AJCC8 only reports MSS, and the risk of metastasis or recurrence are both meaningful endpoints to inform risk-aligned management of melanoma patients. The test’s results can then be reviewed in the context of existing NCCN guidelines that recommend treatments based on risk of recurrence. For patients who have not yet had or will not receive an SLN biopsy, an estimation of their risk of recurrence if an SLN biopsy were to be positive (e.g., stage III disease) is valuable for timely, informed patient care.19
The 31-GEP test makes personalized medicine a reality, effectively moving from broad population-based risk assessment using MSS alone provided by AJCC8 and adding a genetic classifier, the 31-GEP, which provides precise, personalized and clinically validated prognostic information that can improve the accuracy of risk stratification to inform management decisions that clinicians and patients face in the clinic.
Real-world case study 1: tumor biology helps guide a more vigilant treatment and surveillance plan
Take the case of a 34-year-old woman, with a non-ulcerated, 0.6 mm primary malignant melanoma on her arm. With these clinicopathological characteristics, the woman was diagnosed with early-stage invasive melanoma (pT1a) and was not considered for SLNB based on AJCC8 criteria, as her melanoma was considered low risk based on the combination of Breslow depth and ulceration status. Without the 31-GEP test, the recommended treatment plan would likely have been to follow the patient with a history and physical examination every six to 12 months for five years, then annually.
Because of the patient’s young age and a Breslow depth on the thicker end of a thin melanoma (pT1a – 0.1-0.74mm), the dermatology clinician was concerned and was unsure whether to refer the patient to medical oncology. The 31-GEP test was ordered to provide additional guidance on the management plan for this patient.
Figure 4: 31-GEP test results for case study
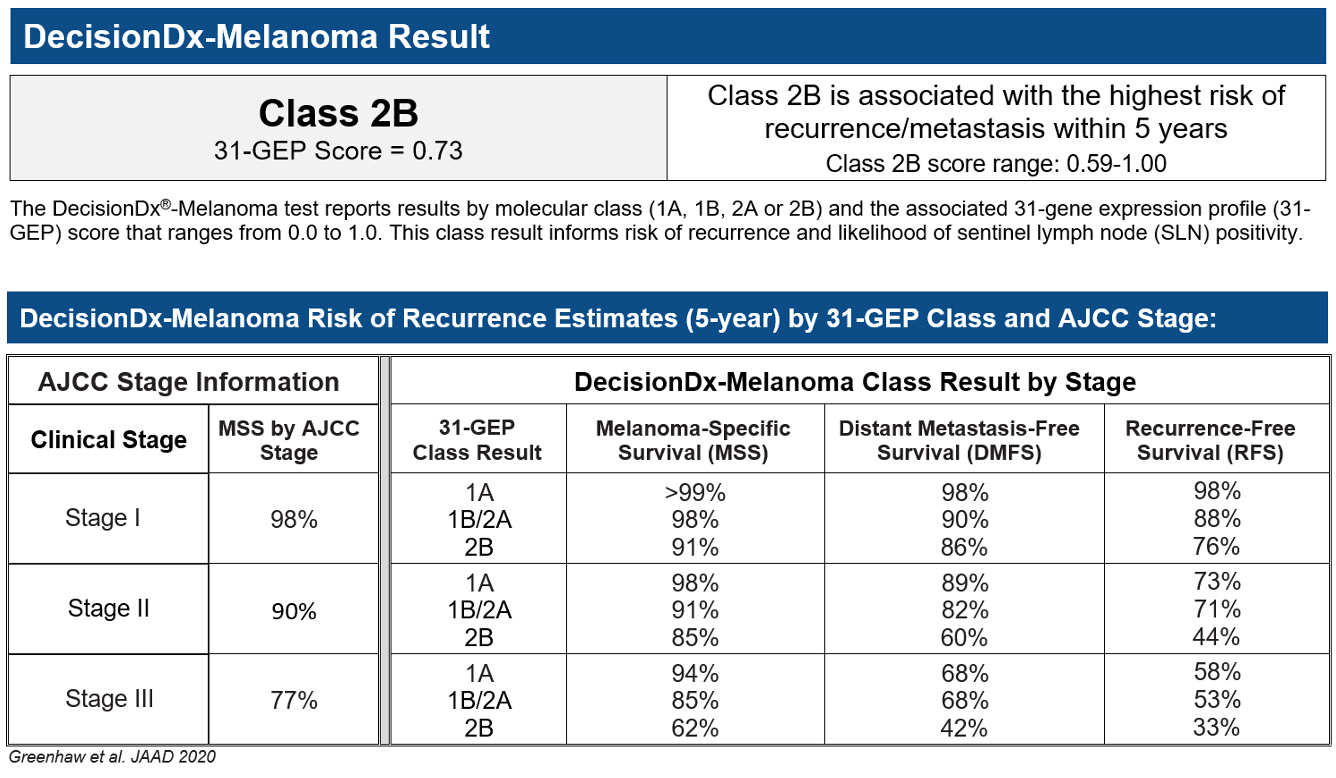
The 31-GEP test returned a Class 2B result, the highest risk result, indicating that the aggressive biology of her melanoma put her at an elevated risk of recurrence and metastasis. Because of the high-risk result, the dermatologist referred the patient to a medical oncologist for high intensity surveillance, with CT scans at baseline and subsequent follow-up. On her second CT scan six months later, the oncologist identified metastasis to the lung. The biopsy showed it was a proven oligomet that was BRAF negative, and the patient was treated with radiotherapy to the lung and started on combination immunotherapy therapy (ipilimumab/nivolumab). The patient is doing well with clear scans more than six years later.
Had the patient’s management been dictated by clinicopathologic factors alone, the outcome of her disease could have been very different. It is very likely that she would not have been referred to medical oncology or obtained surveillance imaging. Moreover, her melanoma metastasis may have been detected symptomatically, and thus she would have received treatment significantly later in her disease course, with a higher tumor burden that may have been more challenging to treat.
A collaboration with NCI and the SEER Program’s registries to advance research and patient care in cutaneous melanoma
Castle recently announced a collaboration with the National Cancer Institute (NCI) to link DecisionDx®-Melanoma (31-GEP) testing data with data from the Surveillance, Epidemiology and End Results (SEER) Program’s registries on CM cases. Through the collaboration, patients who received 31-GEP test reports were linked to those who were entered into the SEER registries’ database and had associated outcomes, such as overall survival (OS) and MSS, using a third-party honest broker for the SEER registries. To assess if patients tested with the 31-GEP had higher survival rates than untested patients, a group of tested patients (n=3,261) was matched to a group of patients who did not receive 31-GEP test results as part of their clinical care (n=10,863); the matching was based on 11 clinicopathologic and socioeconomic variables using a 1:3 ratio, to ensure that patients who had the benefit of both the 31-GEP test and traditional clinicopathologic features were matched or balanced. Matching cases were limited to diagnoses in 2016 and forward to account for potential access to adjuvant therapy.
A study of these real-world, unselected, prospectively tested patients with CM showed that the precise, personalized test results provided by the 31-GEP not only mirrored the risk stratification shown in previously published cohorts, but also provided direct evidence that the test’s results have the potential to improve patient survival when used as part of a melanoma management plan.20-21 In this study, patients diagnosed with melanoma and tested with the 31-GEP had improved survival (27% improvement in MSS and 21% improvement in OS) compared to untested patients, who only had access to traditional clinical and pathologic factors to determine their treatment and follow-up plan. Not only was the 31-GEP test able to accurately and independently risk stratify patients for disease progression, but the data suggests that 31-GEP test results can aid in providing more risk-aligned treatment plans for improved patient outcomes.22
Figure 5: Patients with 31-GEP test results had improved survival

‡ Hazard ratio (HR) was computed using the matched untested patients as reference for 31-GEP tested cohort. An HR less than 1.0 demonstrates improved survival in 31-GEP tested patients. Diagnosis date 2016 and onward.
Scaling up – potential impacts of wide-scale adoption of the 31-GEP test in the clinic
When the benefits of an individualized melanoma risk assessment that accounts for tumor characteristics and biology are scaled to a population, the possible impact extends from improved risk stratification for patients to potential improvements in healthcare costs and patient outcomes.
Referring to 31-GEP test results when considering the next steps in a patient’s treatment can potentially reduce unnecessary surgical procedures often performed on low-risk patients and focus resources on patients with more aggressive cases of melanoma at a higher risk of recurrence, resulting in a better utilization of healthcare resources. Individuals with high-risk tumor biology are also more likely to receive more intensive care and frequent follow-up so that metastasis or recurrence can be identified and treated when the tumor burden is low.23
Current risk stratification, while helpful, has limitations. With the 31-GEP test, clinicians are equipped with additional, personalized information for their patients to ensure that follow-up patient care is informed by tumor biology and clinicopathologic factors. The 31-GEP test has been supported by extensive clinical utility and validity studies, exemplified by the patient story presented here. The test is reimbursed by Medicare for clinical use in the management of patients with melanoma and brings personalized medicine to patients today.

Aaron S. Farberg, M.D.
Aaron S. Farberg, M.D. is a double board-certified dermatologist and Mohs surgeon that specializes in skin cancer and inflammatory disease. Dr. Farberg is a summa cum laude graduate of Emory University and continued his education at the University of Michigan where he received his medical degree. Dr. Farberg’s post graduate training includes additional time at the University of Michigan in plastic and reconstructive surgery and, in New York, as a clinical research fellow in association with the National Society for Cutaneous Medicine and New York University. He then remained in New York, completing a residency in dermatology at the Icahn School of Medicine at Mount Sinai Hospital where he served as chief resident.
In addition to treating patients, Dr. Farberg has been a clinical investigator on several studies including FDA clinical trials. He has been recognized with numerous awards and honors, including taking first place on several occasions at regional and national medical conference competitions. Dr. Farberg is an accomplished author and has published over 100 articles in professional medical journals. While in New York, he served as a consultant dermatologist for the New York Yankees.
References
- American Joint Committee on Cancer. Melanoma of the Skin. In: AJCC Cancer Staging Manual. 8th ed. Springer; 2017. Accessed March 11, 2022. http://link.springer.com/book/9783319406176
- National Comprehensive Cancer Network. Melanoma: Cutaneous, Version 3.2022 - April 11, 2022, in NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). [Internet]. 2022; Available from: https://www.nccn.org/professionals/physician_gls/pdf/cutaneous_melanoma.pdf
- Kashani-Sabet M, Miller JR 3rd, Lo S, et al. Reappraisal of the prognostic significance of mitotic rate supports its reincorporation into the melanoma staging system. Cancer. 2020 Nov 1;126(21):4717-4725. doi: 10.1002/cncr.33088. Epub 2020 Aug 11. PMID: 32780467
- Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27(36):6199-206. doi: 10.1200/JCO.2009.23.4799. Epub 2009 Nov 16. PMID: 19917835; PMCID: PMC2793035.
- Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2017, National Cancer Institute. Bethesda, MD, https://seer.cancer.gov/csr/1975_2017/, based on November 2019 SEER data submission, posted to the SEER web site, April 2020.
- Ibrahim AM, Le May M, Bossé D, et al. Imaging Intensity and Survival Outcomes in High-Risk Resected Melanoma Treated by Systemic Therapy at Recurrence. Ann Surg Oncol. 2020; 27(10): 3683-3691. doi: 10.1245/s10434-020-08407-8.
- Williams A, Hamilton O, Likar C, et al. The benefit of positron emission tomography/computed tomography in stage I and stage II melanomas with high-risk DecisionDx-Melanoma scores. The American Surgeon. 2022;88(7):1446-1451. doi:10.1177/00031348221081760
- Hyams DM, Covington KR, Johnson CE, et al. Integrating the melanoma 31-gene expression profile test to surgical oncology practice within national guideline and staging recommendations. Future Oncol. 2020 doi.org/10.2217/fon-2020-0827
- Arnot SP, Han G, Fortino J, et al. Utility of a 31-gene expression profile for predicting outcomes in patients with primary cutaneous melanoma referred for sentinel node biopsy. Am J Surg 2021; 221: 1195-9.
- Castle Biosciences Scientific References. Scientific References. Castle Biosciences. https://castletestinfo.com/products/decisiondx-melanoma/scientific-references/. Accessed September 26, 2022.
- Wisco OJ, Marson JW, et al. Improved cutaneous melanoma survival stratification through integration of 31-gene expression profile testing with the American Joint Committee on Cancer 8th Edition Staging; Melanoma Research. 2022;32(2):98-102. doi: 10.1097/CMR.0000000000000804
- Greenhaw BN, Covington KR, Kurley SJ, et al. Molecular risk prediction in cutaneous melanoma: a meta-analysis of the 31-gene expression profile prognostic test in 1,479 patients. J Am Acad Dermatol. 2020; 83(3):745-754. doi.org/10.1016/j.jaad.2020.03.053
- MolDX: Melanoma risk stratification molecular testing. CMS.gov Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare-coverage-database/view/lcd.aspx?lcdid=37725&ver=38&proposedStatus=all&sortBy=title&bc=9. Accessed September 26, 2022.
- Whitman ED, Koshenkov VP, Gastman BR, et al. Integrating 31-Gene Expression Profiling with Clinicopathologic Features to Optimize Cutaneous Melanoma Sentinel Lymph Node Metastasis Prediction. JCO Precis Oncol. 2021;(5):1466-1479. doi:10.1200/PO.21.00162
- Jarell A, Gastman BR, Dillon LD, et al. Optimizing treatment approaches for patients with cutaneous melanoma by integrating clinical and pathologic features with the 31-gene expression profile test. J Am Acad Dermatol. 2022. doi: https://doi.org/10.1016/j.jaad.2022.06.1202.
- Dillon LD, McPhee M, Davidson RS, et al. Expanded evidence that the 31-gene expression profile test provides clinical utility for melanoma management in a multicenter study. Curr Med Res Opin. 2022; 38(8): 1267-1274. https://doi.org/10.1080/03007995.2022.2033560.
- Marchetti MA, Coit DG, Dusza SW, et al. Performance of gene expression profile tests for prognosis in patients with localized cutaneous melanoma: a systematic review and meta-analysis. JAMA Dermatol. 2020; 156(9): 953-962. doi: 10.1001/jamadermatol.2020.1731. PMID: 32745161; PMCID: PMC7391179
- Vetto JT, Hsueh EC, Gastman BR, et al. Guidance of sentinel lymph node biopsy decisions in patients with T1-T2 melanoma using gene expression profiling. Future Oncol. 2019; 15(11): 1207-17. https://doi.org/10.2217/fon-2018-0912.
- Grossman D, Okwundu N, Bartlett EK, et al. Prognostic gene expression profiling in cutaneous melanoma: identifying the knowledge gaps and assessing the clinical benefit. JAMA Dermatol. 2020 Sep 1;156(9):1004-1011. doi: 10.1001/jamadermatol.2020.1729. PMID: 32725204; PMCID: PMC8275355.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019; 80(1): 149-157.e4. doi: 10.1016/j.jaad.2018.07.028. Epub 2018 Aug 4. PMID: 30081113
- Zager JS, Gastman BR, Leachman S, et al. Performance of a prognostic 31-gene expression profile in an independent cohort of 523 cutaneous melanoma patients. BMC Cancer. 2018; 18:130. https://doi.org/10.1186/s12885-018-4016-3.
- Kurley S, Bailey C, Martin B, et al. Incorporating the 31-gene expression profile test stratifies survival outcomes and leads to improved survival compared to clinicopathologic factors alone: A Surveillance, Epidemiology, and End Results (SEER) Program collaboration. Poster at 18th European Association of Dermato-Oncology (EADO) Congress: April 21-23, 2022; Seville, Spain.
- Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016; 32 (9): 1599-1604. doi:10.1080/03007995.2016.1192997.
- Schuitevoerder D, Heath M, Cook RW, et al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J Drugs Dermatol. 2018; 17:196-9.
- Scott AM, Dale PS, Conforti A, et al. Integration of a 31-Gene Expression Profile into Clinical Decision-Making in the Treatment of Cutaneous Melanoma. Am Surg. 2020; 86:1561-1564. doi: 10.1177/0003134820939944.
- Marson J, Litchman G, Svoboda R, et al. Assessment of the 31-Gene Expression Profile Test by Dermatologists: A Cross-Sectional Survey from National Dermatology Conferences. J of Skin. 2021; 5:101-107. doi: https://doi.org/10.25251/skin.5.2.4.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.





