- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
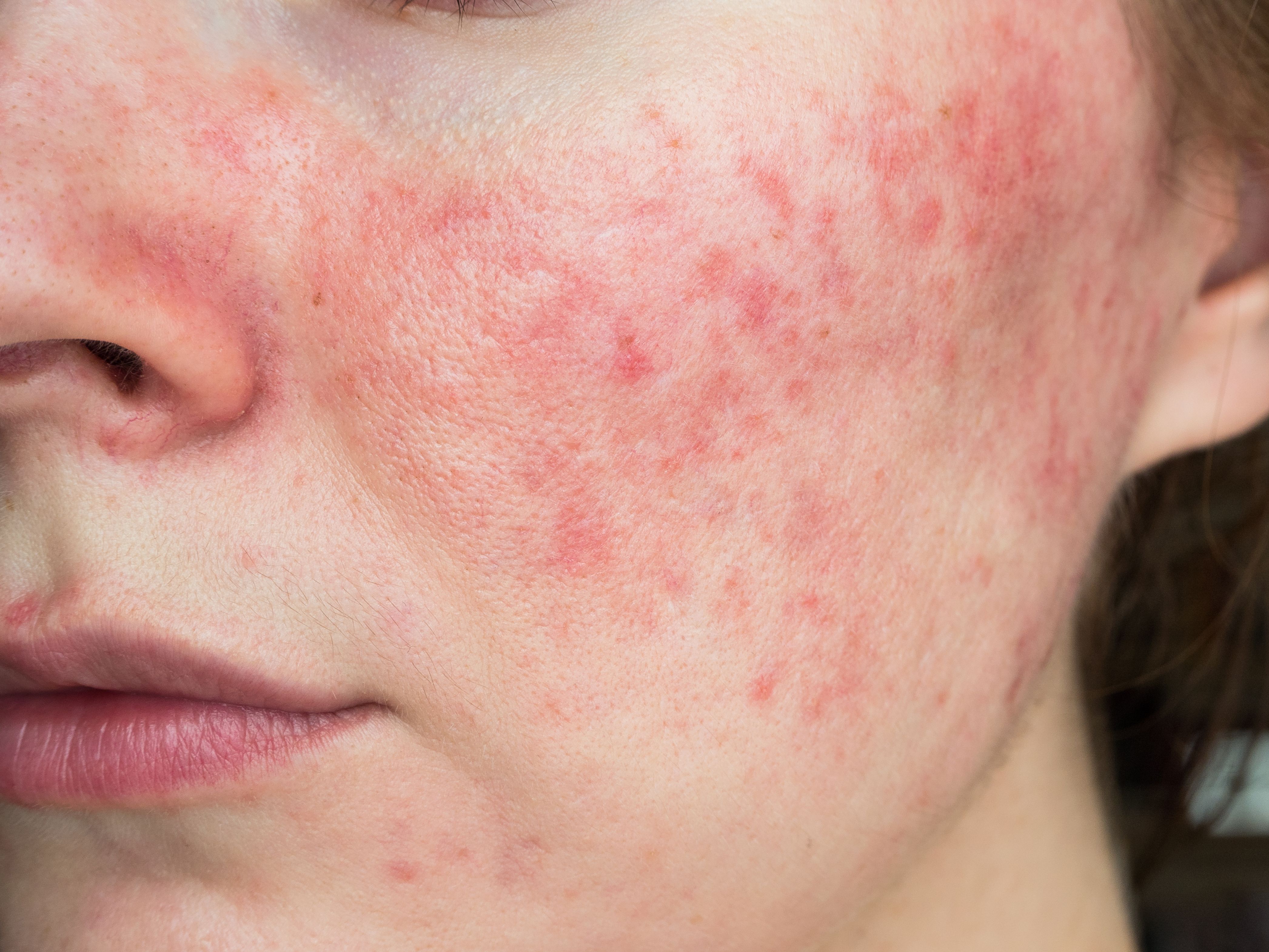
New Study Supports SSA for Rosacea Treatment
Author(s):
Key Takeaways
- Thirty-four patients with PPR were studied, showing significant improvements in lesion reduction and erythema with 30% SSA treatment.
- The experimental group demonstrated a 68.75% efficacy rate, with significant decreases in Investigator Severity Assessment scores and VISIA red area scores.
Patients receiving SSA treatments showed significant improvements after just 2 sessions, supporting its rapid efficacy.
Among rosacea's 4 subtypes, papulopustular rosacea (PPR) is common and is associated with impaired skin barrier function.1 Traditional treatments include topical and systemic antibiotics, retinoids, and beta-blockers, but they often cause adverse events.2 Supramolecular salicylic acid (SSA), a novel formulation, offers potential benefits in treating PPR.3 A recent study investigated the efficacy and safety of 30% SSA as a standalone treatment for PPR, with a focus on lesion reduction, erythema improvement, and skin barrier restoration.4
“Our findings suggest that 30% SSA is an effective and safe option for managing papulopustular rosacea, offering a well-tolerated alternative to traditional treatments,” researchers behind the study wrote.
Materials and Methods
Thirty-four patients with PPR were enrolled in this study between July and December 2020 at the dermatology outpatient clinic of the First Hospital of China Medical University. Inclusion criteria included patients aged 18 to 60 years diagnosed with PPR based on the National Rosacea Society's 2017 diagnostic criteria. Exclusion criteria included other facial skin conditions, recent use of certain medications, pregnancy, and chronic illnesses requiring long-term treatment.
Patients were randomly assigned to either an experimental group receiving 30% SSA treatments every 2 weeks for 4 sessions or a control group receiving a placebo. Efficacy evaluations included lesion counts, Investigator Severity Assessment (ISA) scores, and VISIA red area scores. Skin barrier function was assessed through measurements of trans-epidermal water loss (TEWL), stratum corneum hydration, sebum content, and skin pH. Data were analyzed using GraphPad Prism 8.0, with statistical significance set at p < 0.05.
Results
According to researchers, 31 patients completed the study. They reported the experimental group showed a significant reduction in lesions, with a 68.75% efficacy rate compared to 0% in the control group (p < 0.01). The experimental group also demonstrated a significant decrease in ISA scores (p < 0.01), indicating improved disease severity. The VISIA red area score showed a 25.1% improvement in the experimental group compared to no improvement in the control group (p < 0.01).
Skin barrier function tests revealed decreased TEWL, reduced sebum levels, and improved stratum corneum hydration in the experimental group. The study stated adverse reactions were minimal, with only 1 patient experiencing mild burning and stinging sensations, which did not interfere with treatment. One patient in the control group developed facial erythema, which was managed with antihistamines and pimecrolimus cream.
Discussion
Rosacea's pathophysiology involves impaired skin barrier function, increased TEWL, decreased hydration, and inflammation, forming a cycle that exacerbates symptoms. Salicylic acid is known for its keratolytic, anti-inflammatory, and antimicrobial properties. SSA, a water-soluble stable compound, provides a sustained release of salicylic acid, reducing irritation while enhancing efficacy.
Previous studies suggest SSA's effectiveness when combined with antibiotics or other agents, but limited data exist on its standalone use. This study confirms that 30% SSA effectively reduces lesions, improves erythema, and enhances skin barrier function. Significant improvements were observed after the second treatment session. SSA's ability to stabilize the skin barrier and reduce inflammatory markers supports its role as a potential monotherapy for PPR.
Conclusion
This study demonstrates that 30% SSA is a promising and effective treatment for PPR, offering significant improvements in lesion count, erythema, and skin barrier function with minimal adverse events. The study stated further large-scale studies are warranted to confirm these findings and optimize treatment protocols. Researchers wrote that SSA could provide a valuable alternative to conventional therapies, particularly for patients seeking reduced irritation and enhanced skin health outcomes.
References
- Medgyesi B, Dajnoki Z, Béke G, et al. Rosacea Is characterized by a profoundly diminished skin barrier. J Invest Dermatol. 2020;140(10):1938-1950.e5. doi:10.1016/j.jid.2020.02.025
- Paiva-Santos AC, Gonçalves T, Peixoto D, et al. Rosacea topical treatment and care: From traditional to new drug delivery systems. Mol Pharm. 2023;20(8):3804-3828. doi:10.1021/acs.molpharmaceut.3c00324
- Zhou H, Qiao S, Zhao X, Zeng W. Supramolecular salicylic acid alleviates skin photoaging by increasing collagen density and elasticity. Aesthetic Plast Surg. 2025;49(1):314-321. doi:10.1007/s00266-024-04180-1
- Wang Z, Wu Y, Varkani FN, et al. 30% supramolecular salicylic acid improved symptoms and skin barrier in papulopustular rosacea. J Cosmet Dermatol. 2025;24(2):e70046. doi:10.1111/jocd.70046