- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
New drug application submitted for acne topical
Author(s):
Cassiopea SpA submitted a New Drug Application (NDA) to the U.S. Federal Drug Administration (FDA) for its novel acne treatment, clascoterone cream 1%, according to a company announcement released today.
If approved, clascoterone would be the first new mechanism of action for acne in nearly 40 years. (soupstock - stock.adobe.com)
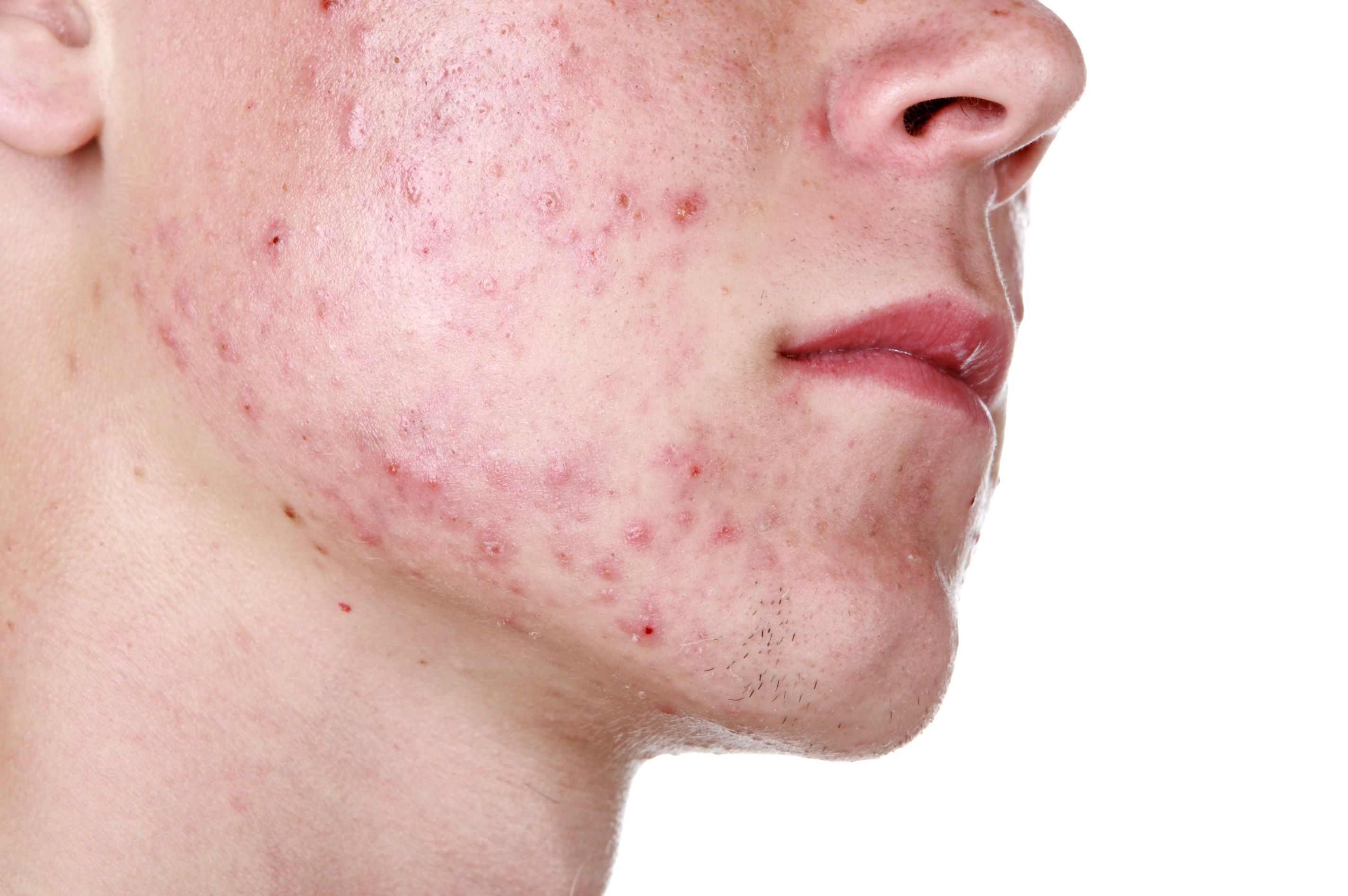
Cassiopea SpA submitted a New Drug Application (NDA) to the U.S. Federal Drug Administration (FDA) for its novel acne treatment, clascoterone cream 1%, according to a company announcement released today.
If approved, clascoterone would be the first new mechanism of action for acne in nearly 40 years. The topical is designed to penetrate the skin and block the androgen receptors of the sebaceous gland without causing systemic side effects.
“This is noteworthy because it’s been so long since there has been a new, first-in-class molecule for the treatment of acne, particularly given that it is a topical treatment that targets the androgen receptor and works on sebocytes to mediate lipid production and inflammation,” said Dr. Lawrence Eichenfield, Chief of Pediatric and Adolescent Dermatology at Rady Children's Hospital–San Diego. “Giving physicians another treatment option for their patients who struggle with acne is tremendously important.”
Topline results from two Phase 3 clinical trials showed statistically significant improvements for all primary endpoints with no treatment related serious adverse events. If local skin reactions did occur, they tended to be similar to vehicle and mostly classified as mild. The safety of the topical was explored in an open-label safety study lasting up to one year. Participants also used the cream on both the face and the trunk. The study found that the increase in duration of use and coverage did not increase the incidence of significant side effects.
“We’re focused on the urgency to treat skin conditions that can leave not only physical scars, but also emotional scars,” says Diana Harbort, CEO of Cassiopea. “That’s why innovation is so critical. We are committed to finding a way to treat acne that addresses the root causes of the condition.”





