- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
National Rosacea Society Launches Seal of Acceptance Program
Alongside expert dermatologists, the NRS recently developed a Seal of Acceptance for skin care and cosmetic products clinically tested and evaluated for patients with rosacea.
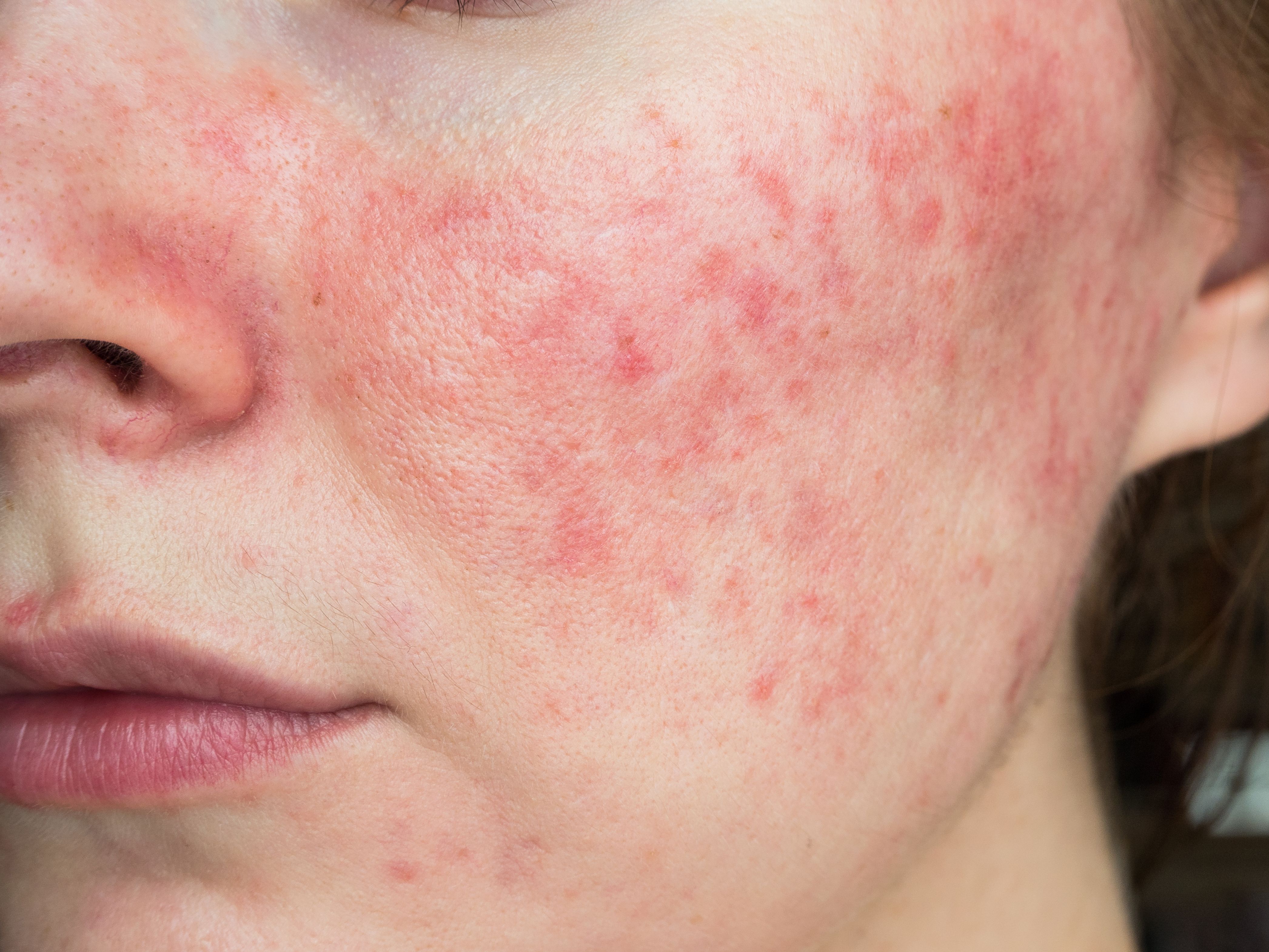
National Rosacea Society (NRS) Seal of Acceptance for skin care and cosmetic products

The National Rosacea Society (NRS) recently announced the official launch of its Seal of Acceptance, an initiative aimed at providing clinicians and patients with the ability to identify skin care and cosmetic products that have been clinically tested and evaluated to be gentle and soothing enough for patients with rosacea.
In previous surveys, the NRS notes that 66% of patients with rosacea reported the ability to name specific skin care or cosmetic products as triggers for symptoms. Furthermore, 84% of patients expressed an interest in guidance regarding skin care product selection and use.
“One of the most common requests the NRS receives from rosacea patients is for information and advice about which skin care and cosmetic products are appropriate to use,” said Andrew Huff, president and executive director of the NRS, in a news release. “We are pleased to offer this resource to rosacea sufferers by recognizing products that have been shown to be unlikely to cause irritation or a flare-up.”
Last year, the NRS announced it was beginning to allow companies to begin applying for the seal for their products. Applicants were asked to provide a full ingredient list as well as data from human repeat insult patch tests performed on at least 30 individuals. Additionally, data from safety-in-use tests in a minimum of 30 patients was required. Products containing ingredients leading to skin barrier dysfunction, vasomotor instability (flushing), or unwanted neurosensory stimulation, such as burning or itching, were not considered for the seal.
The Seal of Acceptance program was developed utilizing guidance from expert dermatologist Zoe Diana Draelos, MD, FAAD. Draelos is a consulting professor of dermatology at Duke University School of Medicine in Durham, North Carolina, president of Dermatology Consulting Services, PLLC, and the Editor in Chief Emeritus of Dermatology Times. She also serves on the board of directors for the NRS.
Draelos recently spoke with Dermatology Times to discuss her involvement in the development of the program, the importance of the initiative for clinicians and patients alike, and more.

Developing Guidelines and Criteria
According to Draelos, product selection is particularly important in patients with rosacea.
"Good selection of skin care products can minimize the signs and symptoms of rosacea, and poorly selected products can make it worse," she said. "Many rosacea patients will come with baskets of things they've tried, and these break them out. This makes their face redder; this makes stinging and burning occur."
Draelos, who has been doing research in rosacea for many years, also has involvement in the skin care industry and has spent time working to design products specifically for sensitive skin.
"The National Rosacea Society asked me if I would put together a document that would be the testing requirements and the other details that needed to be met by a product in order to receive the seal," Draelos said. "It's been a passion of mine for quite some time, and it's exciting to actually see the seal launched."
Expert Involvement
A group of dermatology experts with expertise in rosacea evaluate each of the Seal of Acceptance applications received by the NRS, Draelos said.
With this in mind, experts are involved in every step of the process. They help industry develop the product, test the product, and then review the testing results, formulation, and application materials in order to then make an assessment.
"This is really a dermatologist-driven initiative," Draelos said. "It's really a way of raising the bar [and] trying to raise the standard of skin care products that are suitable for rosacea."
A Step Forward for Clinicians and Patients
"The idea is that if a person with rosacea sees the seal, they'll know that that product has been tested on rosacea sufferers like themselves, and there's a greater likelihood that that product will perform well on their skin, as well," Draelos said. "It's also helpful for the dermatologists, because the dermatologists can tell people: 'The National Rosacea Society is a nonprofit support group for dermatologists, but also for rosacea sufferers. It has this seal, so you know that particular product has been evaluated by a group of dermatologists, tested in the proper patient population, and vetted.'"
Looking Ahead
Draelos expressed excitement about the ability of the seal to provide awareness to product manufacturers and skin care and cosmetic companies. The seal program, she said, provides a link between dermatology and those who make these products.
"This is really a cooperation between dermatologists helping to formulate skin care products for rosacea sufferers and improving the world a little bit," Draelos said. "Everybody brings their best to the table, and then the end result is better products and a more beautiful world. People feel better about their skin. It's just a very positive outcome, and that's exciting."
At the time of this article's publication, 10 products have been granted the NRS's seal, including:
- Double Take Baked Full Coverage Foundation by Laura Geller
- Dermal Repair Cream by Senté
- Gleanser Non-Drying Glycerin Cleanser by Prequel
- Sun Barrier Mineral Broad Spectrum SPF 50 PA++++ by Prequel
- Baked Balance-n-Glow Illuminating Foundation by Laura Geller
- Serica Moisturizing Rosacea Formula by Serica
- Baked Balance-n-Brighten Color Correcting Foundation by Laura Geller
- Universal Skin Solution Dermal Spray Hypochlorous Acid by Prequel
- barrier+ Foaming Oil Cleanser by Skinfix
- Bee Rx Rosacea Rescue by Bee Rx
To view and filter through a list of products that have already received the seal, or to submit a skin care or cosmetic product for the seal program, the NRS encourages companies, clinicians, and consumers to use this link.
Reference
National Rosacea Society launches new Seal Of Acceptance for skin care and cosmetic products. News release. National Rosacea Society (NRS). January 24, 2024. Accessed February 12, 2024. https://www.rosacea.org/blog/2024/national-rosacea-society-launches-new-seal-of-acceptance-for-skin-care-and-cosmetic-products