- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
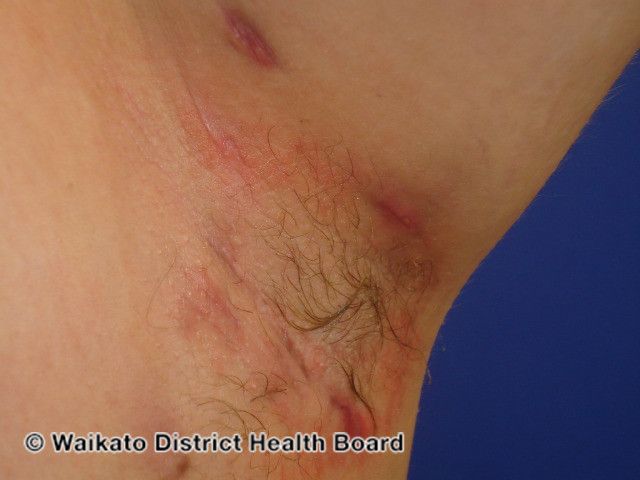
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
MoonLake Immunotherapeutics Announces 24-Week Data of Sonelokimab for HS
Author(s):
After maintenance treatment with sonelokimab dosed every 4 weeks, 57% of patients achieved HiSCR75 at week 24.
MoonLake Immunotherapeutics recently announced new positive 24-week-top-line results from its global phase 2 MIRA trial evaluating the safety and efficacy of sonelokimab, administered subcutaneously, for the treatment of adult patients with moderate to severe hidradenitis suppurativa (HS). Data was presented at the European Academy of Dermatology and Venereology Congress 2023 in Berlin, Germany.1
The MIRA trial is the first placebo-controlled randomized trial in HS to use Hidradenitis Suppurative Clinical Response 75 (HiSCR75) as the primary endpoint.
Key findings include:
- After maintenance treatment with sonelokimab dosed every 4 weeks, 57% of patients achieved HiSCR75 at week 24, increasing the landmark week 12 responses (primary endpoint) by over 10 percentage points (ppt)
- Responses continued to increase from week 12 to week 24 across several scores, including over 14 ppt increases in HiSCR90 and IHS4-90 (to approximately 40% of patients)
- 1 in every 4 patients reached inflammatory remission with IHS4-100, reflecting 100% reduction in abscesses, nodules, and draining tunnels
- Rates of complete resolution of inflammatory nodules and abscesses (AN 100) and draining tunnels (DT 100) increased between weeks 12 and 24
- Clinical responses showed significant improvements in health-related quality of life (DLQI), skin pain (NRS30), and self-reported absent or minimal disease (PGI-S)
- Maintenance dosing, placebo cross-overs to sonelokimab 120mg or 240mg, and pharmacokinetic data confirm 120mg as the dose with optimal benefit-risk profile
- For non-responders to adalimumab at week 12, switching to sonelokimab resulted in HiSCR75 response rates similar to those randomized to sonelokimab at baseline
“The overall data package created with the MIRA trial establishes MoonLake’s position as a leading innovator in the Immunology and Inflammation (I&I) and IL-17 space. We have consistently observed best-in-class clinical activity with our Nanobody® sonelokimab for hidradenitis suppurativa and these results demonstrate its effect on a number of clinically meaningful endpoints. In June we elevated the bar for clinical response to HiSCR75 as the primary endpoint,” said Jorge Santos da Silva, PhD, founder and chief executive officer at MoonLake, in the news release. “We have now advanced to demonstrating even deeper responses (such as HiSCR90, AN 100, and DT 100) and on scores that are important for patients, such as complete inflammatory remission with IHS4-100 and absent or minimal disease activity as reported by patients (PGI-S). Importantly, the results have confirmed our view that 120mg is the optimal dose and we are on track to discuss our Phase 3 development plans with the FDA by the end of this year.”
Overall, the safety profile of sonelokimab was consistent with previous studies, and no new safety signals were observed.
According to MoonLake, “Based on the efficacy and safety results, Q4W maintenance dosing of sonelokimab 120mg has been confirmed in the Company’s view as the optimal dose, in terms of speed and depth of response, and overall benefit-risk profile, for progression into phase 3 development."
MIRA is a global, randomized, double-blind, placebo-controlled trial that enrolled 234 patients. Two different doses of sonelokimab (120mg and 240mg) were evaluated and compared with placebo control and adalimumab as an active reference arm. Secondary end points of the study included the proportion of patients achieving HiSCR50, the change from baseline in International Hidradenitis Suppurativa Severity Score System (IHS4), the proportion of patients achieving a Dermatology Life Quality Index (DLQI) total score of ≤5, and the proportion of patients achieving at least 30% reduction from baseline in Numerical Rating Scale (NRS30) in the Patient’s Global Assessment of Skin Pain (PGA Skin Pain).
Sonelokimab (M1095) is an investigational ~40 kDa humanized Nanobody with 3 VHH domains covalently linked by flexible glycine-serine spacers. According to the news release, with 2 domains, sonelokimab selectively binds with high affinity to IL-17A and IL-17F, thereby inhibiting the IL-17A/A, IL-17A/F, and IL-17F/F dimers. A third central domain binds to human albumin, facilitating further enrichment of sonelokimab at sites of inflammatory edema.
Reference
- MoonLake Immunotherapeutics announces the full dataset from its 24-week MIRA clinical trial, establishing the nanobody sonelokimab as a highly promising and differentiated therapeutic solution for hidradenitis suppurativa. MoonLake Immuotherapeutics. News release. October 15, 2023. Accessed October 16, 2023. https://ir.moonlaketx.com/news-releases/news-release-details/moonlake-immunotherapeutics-announces-full-dataset-its-24-week