- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
News
Article
Majority of Dermatologists in US Prescribe Baricitinib to Patients With Alopecia Areata, Report Shows
Author(s):
Baricitinib is more commonly prescribed by dermatologists for patients with alopecia in the US and is projected to maintain a substantial market lead over ritlecitinib.
Alex Papp/Adobe Stock
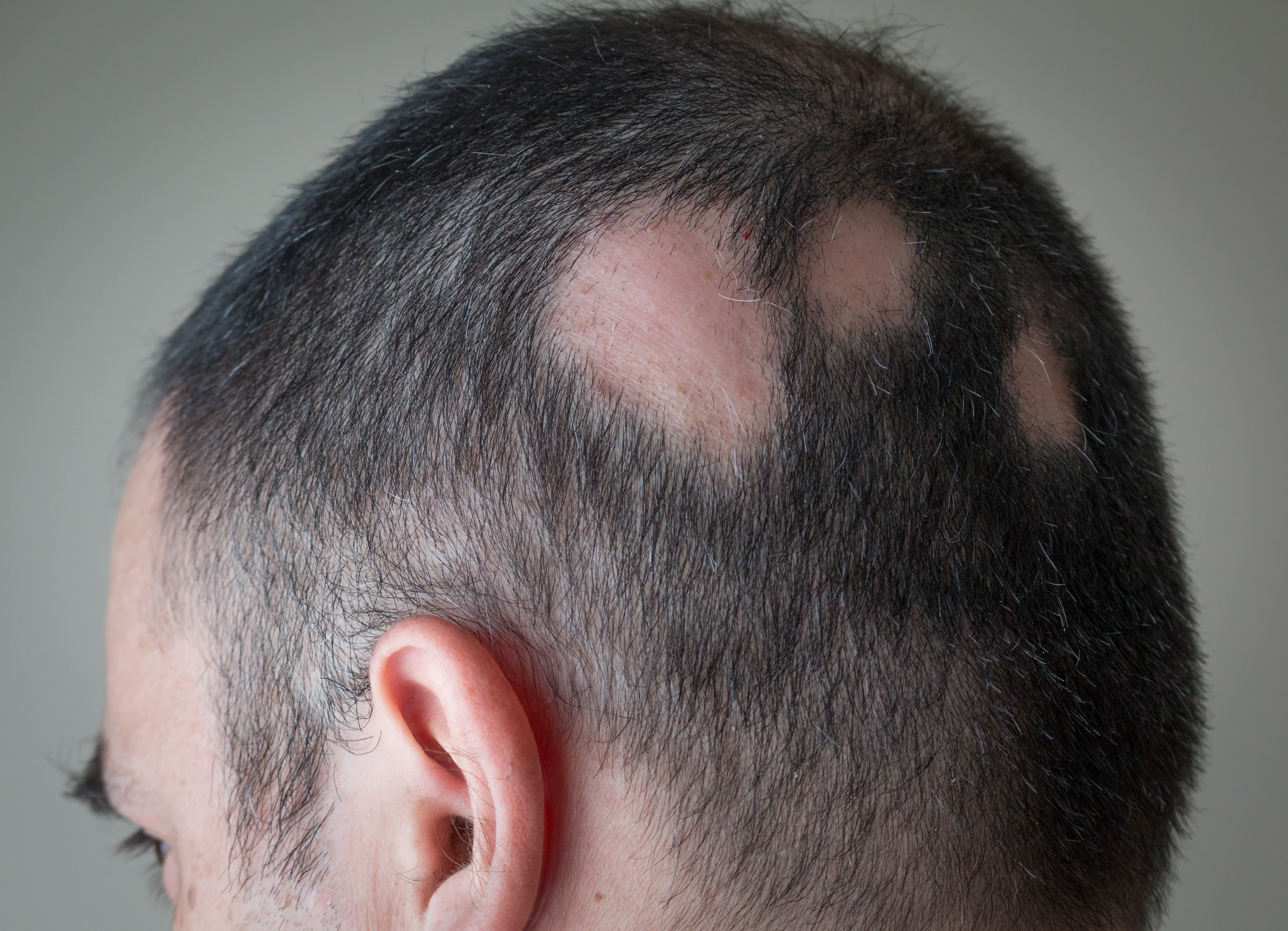
Bariticinib (Olumiant; Eli Lilly) is prescribed by the majority of US dermatologists to their patients with alopecia areata (AA), according to a recent analysis1 (Market Dynamix: Alopecia Areata). Additionally, more than half of US dermatologists have begun trial of ritlecitinib (Litfulo; Pfizer) in their patients with AA.
According to the analysis, 6-month market projections for both drugs are favorable. However, bariticinib is expected to maintain a substantial market lead over ritlecitinib.
Spherix Global Insights notes that within the past 4 years of their analysis of the AA market, the introduction of Janus kinase (JAK) inhibitors has made a significant impact on the market itself, particularly attributable to its ability to meet an unmet need in severe alopecia.
The analysis found that 105 US dermatologists currently prescribe baricitinib to their patients with AA, potentially attributable to baricitinib's earlier introduction in the JAK inhibitor space for AA management.
A specific analysis of 520 AA patient charts reaffirmed this prescription rate, with prescribers noting the choice to prescribe baricitinib could mostly be attributable to familiarity and comfort. The most common factor for initiating treatment with ritlecitinib, the analysis found, was a prescriber and patient desire to trial a newer therapy.
Despite the difference in the number of dermatologists prescribing bariticinib versus ritlecitinib for patients with AA, the analysis found that prescribers of both drugs expressed comparable and high levels of satisfaction. In fact, these rates of satisfaction were significantly greater than rates for non-advanced systemic treatments.
Both drugs received equally favorable evaluations of overall patient satisfaction, eyebrow and eyelash regrowth, and Severity of Alopecia Tool scoring.
"Recent data from Spherix's published studies highlight minimal differentiation among prescribers regarding the two JAK inhibitors," according to Spherix.1 "A significant portion—two-fifths—explicitly state no differentiation between the two, while one-quarter of the remaining respondents emphasize that dosing represents the most notable contrast. Fewer than one-fifth report differences in access and efficacy between the brands."
The analysis also found that for manufacturers of both drugs, "Dermatologists expressed a shared need for support in access/improvement of patient assistance programs, increased provision of samples, enhanced resources for patient and physician education, and an overall demand for more comprehensive data."
Despite these expressed needs, Spherix found that prescribers were generally unaware of the AA drug pipeline. The majority of prescribers involved in the analysis were not able to recall other AA treatments aside from JAK inhibitors in development but were able to recall well-known line-extensions such as upadacitinib (Rinvoq; AbbVie) and deucravacitinib (Sotyktu; BMS) when prompted.
The top drugs currently in development ranked by prescribers based on familiarity included deuruxolitinib (SUN Pharmaceuticals), etrasimod (Pfizer), bempikibart (Q32 Bio/Amgen (Horizon)), and ivarmacitinib (Arcutis).
Less familiar to prescribers were novel therapies currently in development, including daxdilimab (Amgen (Horizon)), farudostat (Aslan), and EQ101 (Equillium).
Reference
- Spherix Global Insights. Eli Lilly’s Olumiant and Pfizer’s Litfulo strengthen alopecia areata arsenal, yet resounding opportunity for JAK expansion and new assets in development remain. GlobeNewswire News Room. November 15, 2023. Accessed November 16, 2023. https://www.globenewswire.com/news-release/2023/11/15/2781240/0/en/Eli-Lilly-s-Olumiant-and-Pfizer-s-Litfulo-Strengthen-Alopecia-Areata-Arsenal-Yet-Resounding-Opportunity-for-JAK-Expansion-and-New-Assets-in-Development-Remain.html





