- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Lipodissolve remains controversial
Author(s):
Dissolving away fat seems like a dream come true for a lot of people. It's also the subject of contoversy. Lipodissolve has been banned in Brazil and almost banned in Kansas. Questions arise about the efficacy and safety of the fat-dissolving technique, yet doctors who do the procedure regularly contend the fat-dissolving procedure is an excellent addition to their fat loss armamentarium.

Questions arise about the efficacy and safety of the fat-dissolving technique, yet doctors who do the procedure regularly contend the fat-dissolving procedure is an excellent addition to their fat-loss armamentarium.
Lipodissolve is on the cutting edge of fat-reduction therapies. Less invasive than liposuction, it relies on the injection of a combination of phosphatidylcholine and deoxycholate into the body.
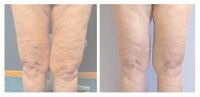
"They are used as drugs in Europe to break up fat clots around the heart. When you combine them, they constitute a drug-like effect, but the FDA (Food and Drug Administration) does not control each individual ingredient in the product," Dr. Torok tells Dermatology Times.
ANVISA, Brazil's version of the FDA, banned importing, making or using any product cosmetically that contains phosphatidylcholine, one of the active ingredients in lipodissolve. An agency news release notes that the product was not licensed in Brazil, and "the banning was justified by the lack of information about the safety of the product in the dissolution of localized fat."
Dr. Torok suggests that problems occurred more because of the therapists than the therapy.
"Brazil couldn't control who was using it. Cosmetics is a culture in Brazil. Completely unqualified people were performing the procedure. Of course, there were complications," she says.
The ruling in Kansas
Then, there was an attempt by the Kansas State Board of Healing Arts (KSBHA) to ban lipodissolve.
Plastic surgeon Diane Duncan, M.D., F.A.C.S., in Fort Collins, Colo., reports that effort ran into opposition.
"Complaints to the Better Business Bureau in Kansas included reports of nodularity, nausea and less-than-desired cosmetic improvement. The nodularity is expected, because the dead fat is not suctioned out. In most cases, it's a temporary situation.
"The great majority of nodules resolve by three months following the injections. Many FDA-approved medications have a side effect of nausea, including chemotherapy," Dr. Duncan says.
In August 2007, a Kansas district court blocked implementation of the regulation in response to a lawsuit filed by Fig, a St. Louis company that operates clinics in several states, which perform lipodissolve. The judge cited a lack of public hearings before approving the regulation.
The KSBHA approved a new regulation in December 2007 to control the use of lipodissolve in the state until more information on its effectiveness, side effects and complications can be compiled.
According to Mark Stafford, general counsel for the KSBHA, physicians will be able to perform lipodissolve if they follow certain guidelines. Physicians must inform the KSBHA of their intent to perform lipodissolve, associate with an institutional review board (IRB) and have plan of care and written informed patient consent approved by the IRB.
Further, physicians must report clinical results to IRB monthly, and report any adverse effect immediately to KSBHA and IRB. Compounding pharmacies must certify sterility and homogeneity of the drug, correctness mixture.
Fig, formerly known as Advanced LipoDissolve, announced that it was filing for Chapter 11 bankruptcy the same day the new regulation was approved by the state board.





