- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
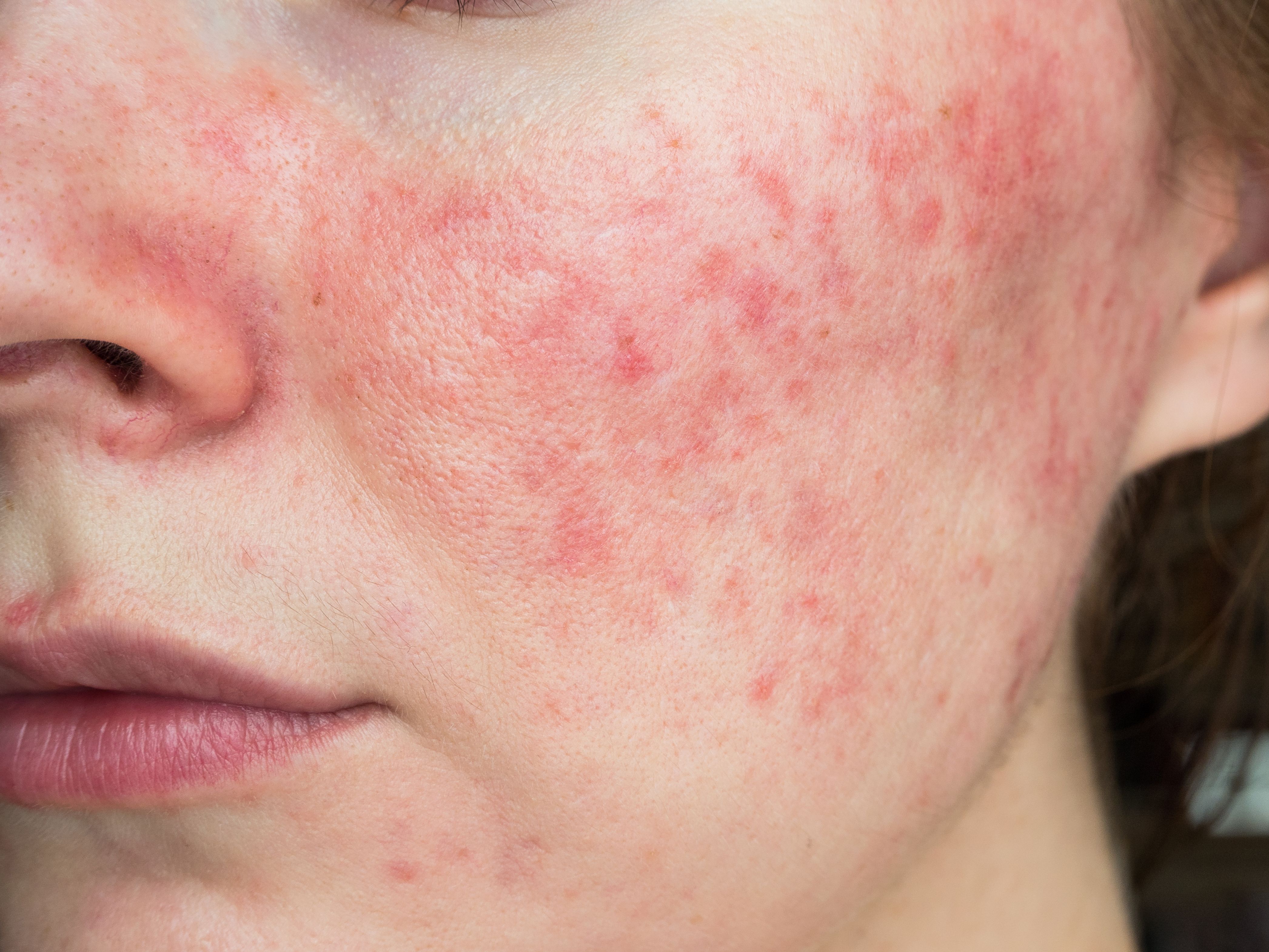
Journey Medical, Dr. Reddy’s to Commercialize and Develop DFD-29 for Rosacea
Author(s):
Journey Medical Corporation and Dr. Reddy’s Laboratories Ltd. have joined forces for the development and commercialization of DFD-19 as a treatment for rosacea.
Journey Medical Corporation, a partner company of Fortress Biotech, Inc, has announced an agreement with Dr. Reddy’s Laboratories Ltd. for the collaboration in development and commercialization of the DFD-29 program (minocycline modified release capsules 40 mg) for the treatment of rosacea.1 Journey Medical acquired global commercialization rights in the U.S. and Europe from Dr. Reddy’s, and Dr. Reddy’s has retained rights to the program in select markets including Brazil, Russia, India, and China.
Both companies will work together to complete the development of DFD-29 and Dr, Reddy’s will monitor 2 future phase 3 clinical trials.
“DFD-29 has the potential to be an effective treatment option for those patients who suffer from rosacea. This collaboration with Dr. Reddy’s for the development of DFD-29 marks a meaningful milestone for Journey Medical, allowing us to continue to broaden our footprint in dermatology while expanding our focus to include late-stage development programs that have a strategic fit,” said Claude Maraoui, president and CEO of Journey Medical, Scottsdale, Arizona.
A completed phase 2 (NCT03340961) study, which enrolled 205 male and female patients diagnosed with rosacea in Germany, evaluated the efficacy, safety, and tolerability of DFD-29 for the treatment of inflammatory lesions of rosacea over 16 weeks.
DFD-29 demonstrated statistical significance over both placebo and active control, Oraycea (doxycycline, Galderma), the German equivalent of U.S. marketed Oracea, on both co-primary endpoints. The endpoints were the proportion of subjects with Investigator’s Global Assessment (IGA) treatment grade 0 or 1 with at least a 2-point grade reduction from baseline at week 16 and total inflammatory lesion count reduction from baseline to week 16. The DFD-29 had about double the efficacy when compared against doxycycline for both endpoints.
“At Fortress and our partner companies, we always seek opportunities to deliver potential medicines to patients in need. We look forward to working with Dr. Reddy’s to further develop DFD-29 for the treatment of rosacea, which has already seen promising results in phase 2 clinical trials. The National Rosacea Society estimates 16 million U.S. based patients suffer from rosacea, demonstrating the sizeable market for this opportunity,” said Lindsay A. Rosenwald, MD, Fortress’ chairman, president and CEO, New York City, New York.
Reference:
1. Inc FB. Journey medical corporation enters into a definitive agreement with dr. Reddy’s laboratories ltd. To develop and commercialize dfd-29 for the treatment of rosacea. GlobeNewswire News Room. Published June 30, 2021. Accessed July 8, 2021. https://www.globenewswire.com/news-release/2021/06/30/2255612/28889/en/Journey-Medical-Corporation-Enters-into-a-Definitive-Agreement-with-Dr-Reddy-s-Laboratories-Ltd-to-Develop-and-Commercialize-DFD-29-for-the-Treatment-of-Rosacea.html