- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Ixekizumab offers quick, lasting relief for genital psoriasis
Author(s):
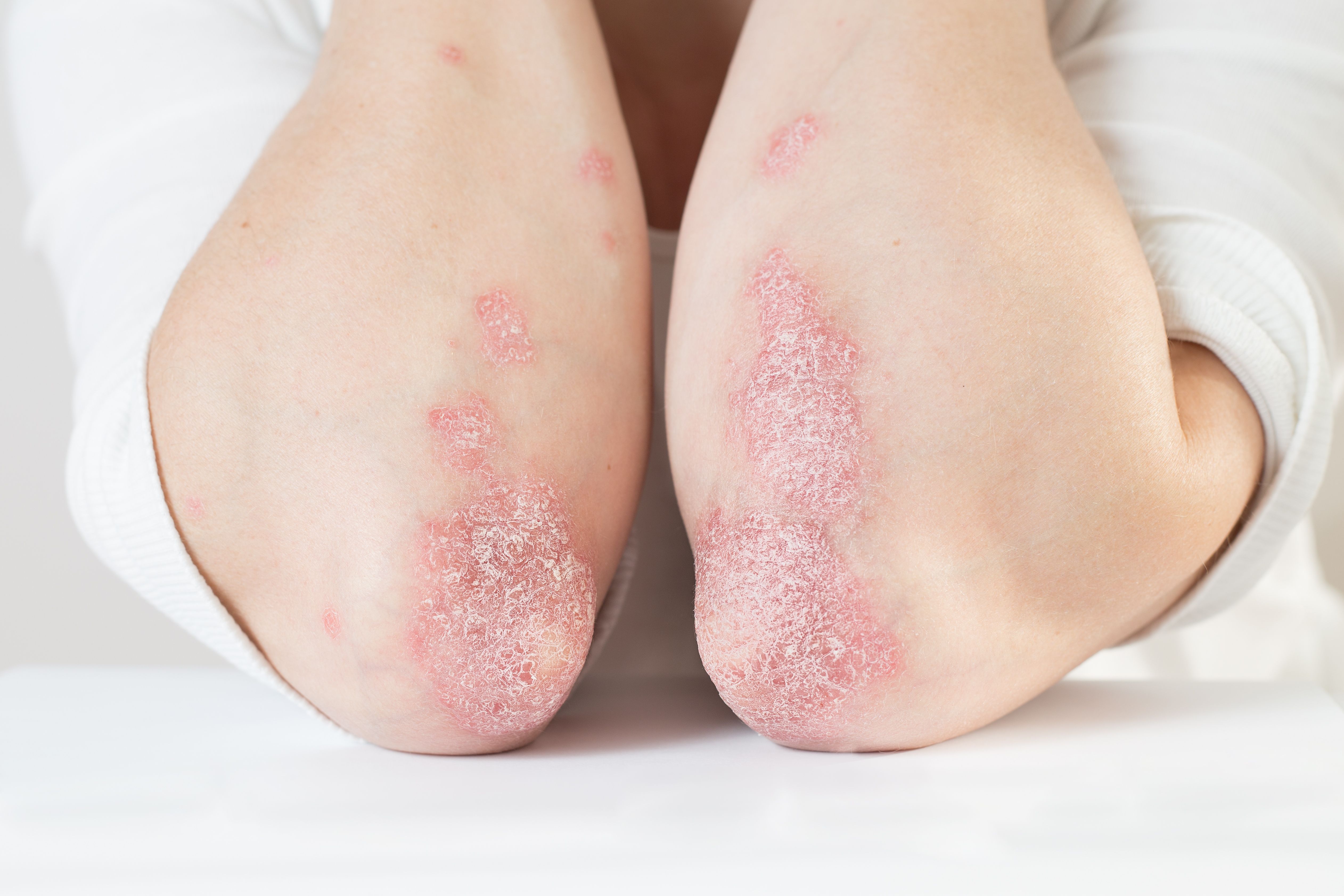
A recent study shows ixekizumab may be able to help treat moderate-to-severe cases of genital psoriasis.
Treatment with ixekizumab (Taltz, Eli Lilly and Company) resulted in quick and lasting relief of genital psoriatic lesions, genital pain and improved sexual health in patients with moderate-to-severe genital psoriasis, according to a 12-week study published January 9, 2020 in the Journal of the European Academy of Dermatology and Venereology.
RELATED: Quality of life suffers in patients with genital psoriasis
An international group of researchers did a post hoc subgroup analysis of the IXORA-Q randomized, placebo-controlled study. IXORA-Q is a study of 149 adult subjects with moderate-to-severe genital psoriasis with a static Physician’s Global Assessment of Genitalia score of three or more and body surface area involvement of 1% or greater. Seventy-five patients received 80 mg of subcutaneous ixekizumab, a high-affinity anti-interleukin-17A monoclonal antibody, every two weeks, following an initial dose of 160 mg, while 74 received placebo for the 12-week blinded treatment.
In the post hoc analysis, researchers looked at clinical response to the active versus placebo as well as the impact of genital psoriasis lesion secondary surface changes, including erosions, fissures and/or ulcers, on genital pain and sexual health.
They found surface disruption signs in genital psoriasis are common. Fifty-seven of the 149 patients, or 38%, presented at baseline with genital erosions, fissures and/or ulcers. Most of those patients had genital fissures, followed by erosions and ulcers.
Secondary lesional changes were associated with significantly higher scores for disease severity and pain but not for sexual health. In the ixekizumab treatment group, 62% of patients had complete resolution of erosions, fissures and/or ulcers in the first week, compared to 25% of the placebo group. Eighty-three percent of patients treated with ixekizumab had complete resolution of the signs at week 12, compared to 21% of patients treated with placebo.
Patients in the ixekizumab group reported significant improvements in pain, itch, disease severity and sexual health compared to those in the placebo group. That was whether or not they had secondary lesional signs at baseline.
“According to our data, signs of surface disruption have a greater impact on patient-reported measures in patients with limited [body surface area] involvement (<10%), highlighting the importance of assessing disease severity and treatment outcomes from both physician and patient perspectives,” the authors write.
RELATED: Systemic nonbiologic therapies guidelines reflect continued popularity of oral treatments
Researchers have found that up to 63% of patients with plaque psoriasis have genital involvement at some point. And the signs of genital psoriasis are often painful and pruritic. Genital psoriasis can impact patients’ sexual health and quality of life. Yet, proven treatments for genital psoriasis are limited.
Genital psoriasis often is under-reported, under-diagnosed and under-treated, according to the authors.
Ixekizumab is approved by the U.S. Food and Drug Administration for the treatment of plaque psoriasis. Systemic therapies are recommended when other treatment options fail for genital psoriasis, according to the paper.
While this analysis is limited by its duration and small number of patients, the authors wrote, the data offer evidence of the profound impact genital psoriasis has on patients and may increase awareness and rates of diagnosis, monitoring and treatment for this disease.
Disclosures:
Eli Lilly and Company funded the study. The authors include consultants, investigators, employees and shareholders of Eli Lilly.
Reference:
Merola JF, Ghislain PD, Dauendorffer JN, et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. J Eur Acad Dermatol Venereol. 2020;34(6):1257-1262.