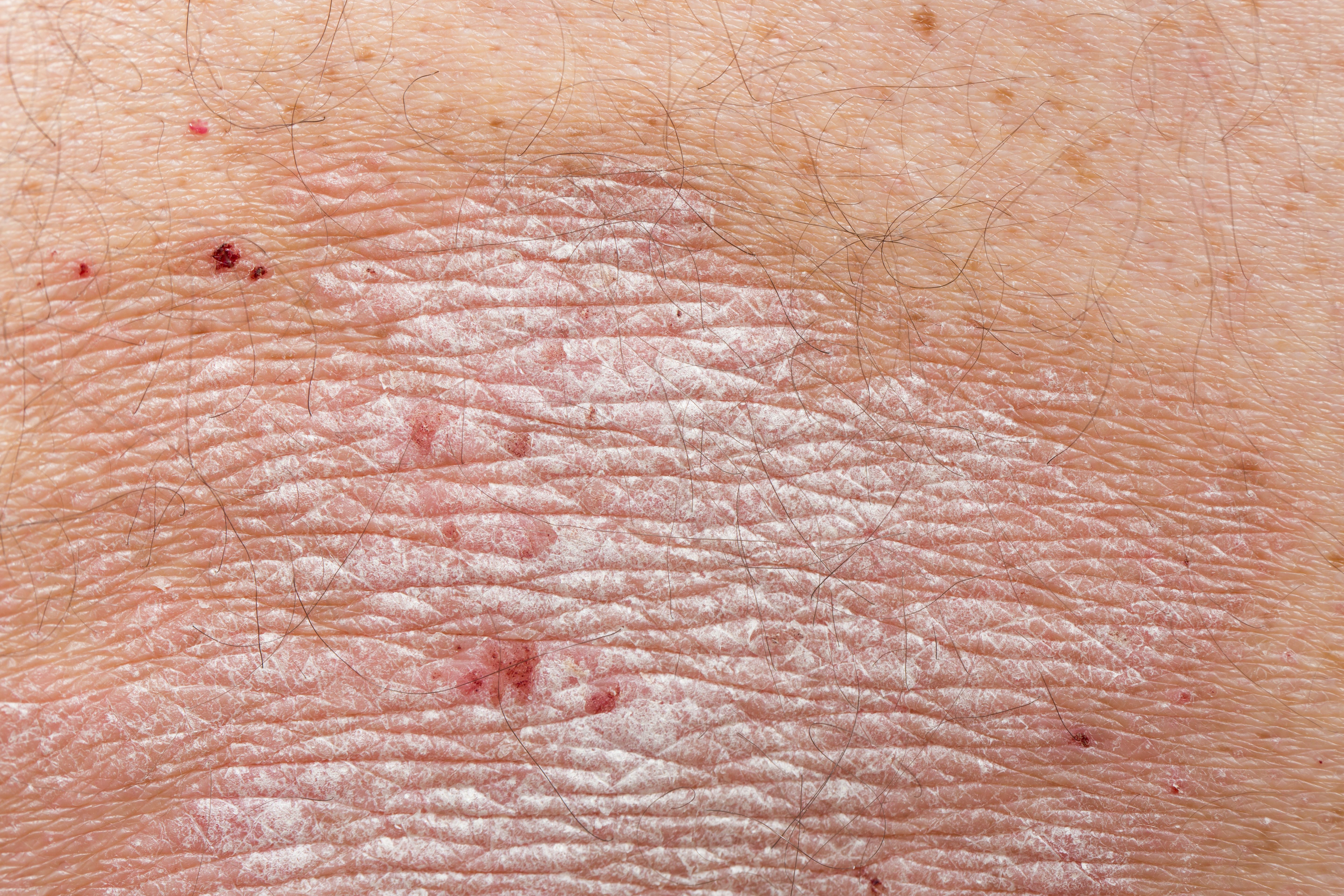
Almirall has unveiled1 positive interim data from its POSITIVE study at the ongoing European Association of Dermatology and Venereology (EADV) Congress in Berlin, revealing that tildrakizumab significantly improved the well-being, health-related quality of life, and more for patients with moderate-to-severe psoriasis and their families.
The POSITIVE study is the first clinical study in dermatology to consider patient well-being a primary endpoint. The study is ongoing and has currently enrolled 780 adults with moderate-to-severe psoriasis spanning several European countries.
At study baseline, approximately 40% of psoriasis patients exhibited depressive symptoms. After 16 weeks of treatment with tildrakizumab, patients achieved significant improvements to their overall well-being comparable to that of the general population. Results were sustained up to week 28 of the study.
"This study demonstrates for the first time the impact of patient's psoriasis on the social and emotional wellbeing of their relatives, underscoring the existing unmet needs not only in the management of psoriatic patients but also their families," according to a press release from Almirall.1
Key Takeaways
- Almirall's POSITIVE study reveals that tildrakizumab significantly improved the well-being and health-related quality of life for patients with moderate-to-severe psoriasis and their families.
- This is the first clinical study in dermatology to consider patient well-being as a primary endpoint.
- After 16 weeks of treatment, patients achieved significant improvements in overall well-being, comparable to that of the general population, with results sustained up to week 28 of the study.
Through week 28, tildrakizumab also demonstrated efficacy with improvements to health-related quality of life and treatment satisfaction. The POSITIVE study will continue to follow patient outcomes through 24 months of treatment with tildrakizumab.
“Psoriasis is a challenging disease that affects not only the skin but also the overall wellbeing of patients. Understanding the broader implications of psoriasis is a significant advance in our field and underlines the importance of a holistic approach to its treatment," said Ulrich Mrowietz, MD, in the press release.1 Mrowietz is a professor of dermatology and the founder of the Psoriasis Center at the University Medical Center Schleswig-Holstein.
"The POSITIVE study represents an important step forward in our understanding of psoriasis by assessing the effect on the wellbeing of patients, their families and healthcare professionals," Mrowietz said.
At the EADV Congress, Almirall also presented positive data from 2 of its other trials in psoriasis, TRIBUTE (NCT04229836) and TILOT. The TRIBUTE study demonstrated improvements to patient-reported outcomes such as sleep quality and work productivity as a result of tildrakizumab use. TILOT study results demonstrated tildrakizumab's efficacy over a 2-year period with improvements made in every measurable parameter, including quality of life and treatment satisfaction.
“The new data presented at the EADV highlight the unmet needs not only in the treatment of psoriatic patients, but also the impact on their families," said Almirall Chief Medical Officer Volker Koscielny, MD, MBA.1 "These data demonstrate the consolidated effectiveness and safety of tildrakizumab in treating plaque psoriasis and patients’ overall wellbeing."
Reference
- Almirall. Almirall’s Ilumetri (tildrakizumab) significantly improves wellbeing for patients and their relatives in moderate-to-severe plaque psoriasis. Almirall. October 12, 2023. Accessed October 12, 2023. https://www.almirall.it/web/almirall.com/newsroom/news/almiralls-ilumetri-tildrakizumab-significantly-improves-wellbeing-for-patients-and-their-relatives-in-moderate-to-severe-plaque-psoriasis.