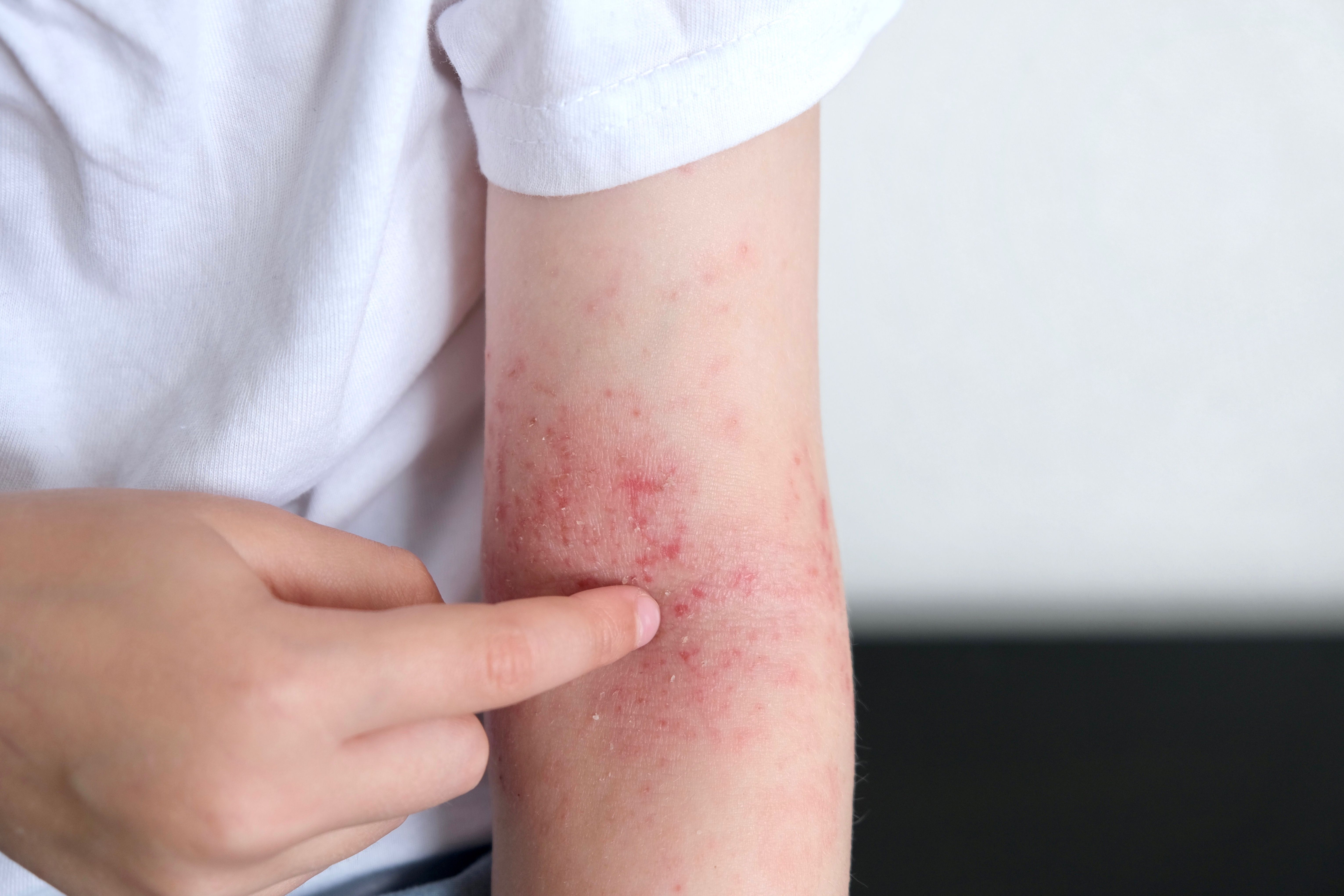
Incyte recently announced1 expanded data from its TRuE-AD3 study of ruxolitinib cream in children between the ages of 2 and 12 years old with atopic dermatitis. Data from the phase 3 study was presented at the European Academy of Dermatology and Venereology (EADV) Congress last week.
In July, Incyte first announced new positive topline data from the TRuE-AD3 study. The randomized, vehicle-controlled, phase 3 trial met the primary endpoint of a set proportion of patients achieving an Investigator’s Global Assessment Treatment Success (IGA-TS), defined as an IGA score of 0 (clear) or 1 (almost clear) with at least a 2-point improvement from baseline at week 8.
Read Dermatology Times' coverage of the TRuE-AD3 study here.
In this expanded data, significantly more patients treated with ruxolitinib 0.75% and 1.5% achieved IGA-TS compared to patients receiving a non-medicated vehicle control cream.
Key Takeaways
- Phase 3 trial met the primary endpoint with a significant proportion of patients achieving Investigator’s Global Assessment Treatment Success (IGA-TS) after 8 weeks of treatment, indicating clearance or almost clear skin.
- Expanded data showed that a higher percentage of patients treated with ruxolitinib cream achieved IGA-TS, along with meeting secondary endpoints, offering a promising non-steroidal topical treatment option for pediatric patients with atopic dermatitis.
Secondary endpoints were also met in the study, including a proportion of patients achieving at least a 75% improvement in Eczema Area and Severity Index by week 8, and time taken to achieve an equal to or greater than 4-point improvement in itch Numerical Rating Scale (NRS4) score.
“AD is a chronic immune-mediated disease that impacts about 13 percent of children in the US, yet there remains a need for new treatment options to help this age group manage this difficult to treat skin condition,” said Lawrence Eichenfield, MD, chief of pediatric and adolescent dermatology at Rady Children’s Hospital San Diego.1 “As a clinician, I have been extremely pleased with the results achieved by many of my adolescent and adult patients with AD prescribed ruxolitinib cream, and I am excited about the potential to have a safe, well tolerated and effective non-steroidal topical treatment option available to my pediatric patients in the future.”
Furthermore, more than half (54%) of patients achieved an IGA score of 0 or 1, indicative of complete or near complete clearance. Nearly 3 out of 4 (73%) achieved NRS4 by as early as week 4.
Treatment emergent adverse events were mild in nature. None led to treatment discontinuation, and none were indicative of systemic JAK inhibition.
"The TRuE-AD3 data presented today at EADV reinforce the strong safety and efficacy profile of ruxolitinib cream and its potential to treat younger age groups,” said Jim Lee, MD, in a press release from Incyte.1 Lee is the group vice president of inflammation and autoimmunity at Incyte.
“There is still a significant medical need for a nonsteroidal topical treatment that provides rapid and effective control of the signs and symptoms of AD in children," Lee said.
Reference
- Incyte announces new data for ruxolitinib cream (Opzelura) in children with atopic dermatitis. Incyte. October 13, 2023. Accessed October 13, 2023. https://investor.incyte.com/news-releases/news-release-details/incyte-announces-new-data-ruxolitinib-cream-opzelurar-children