- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
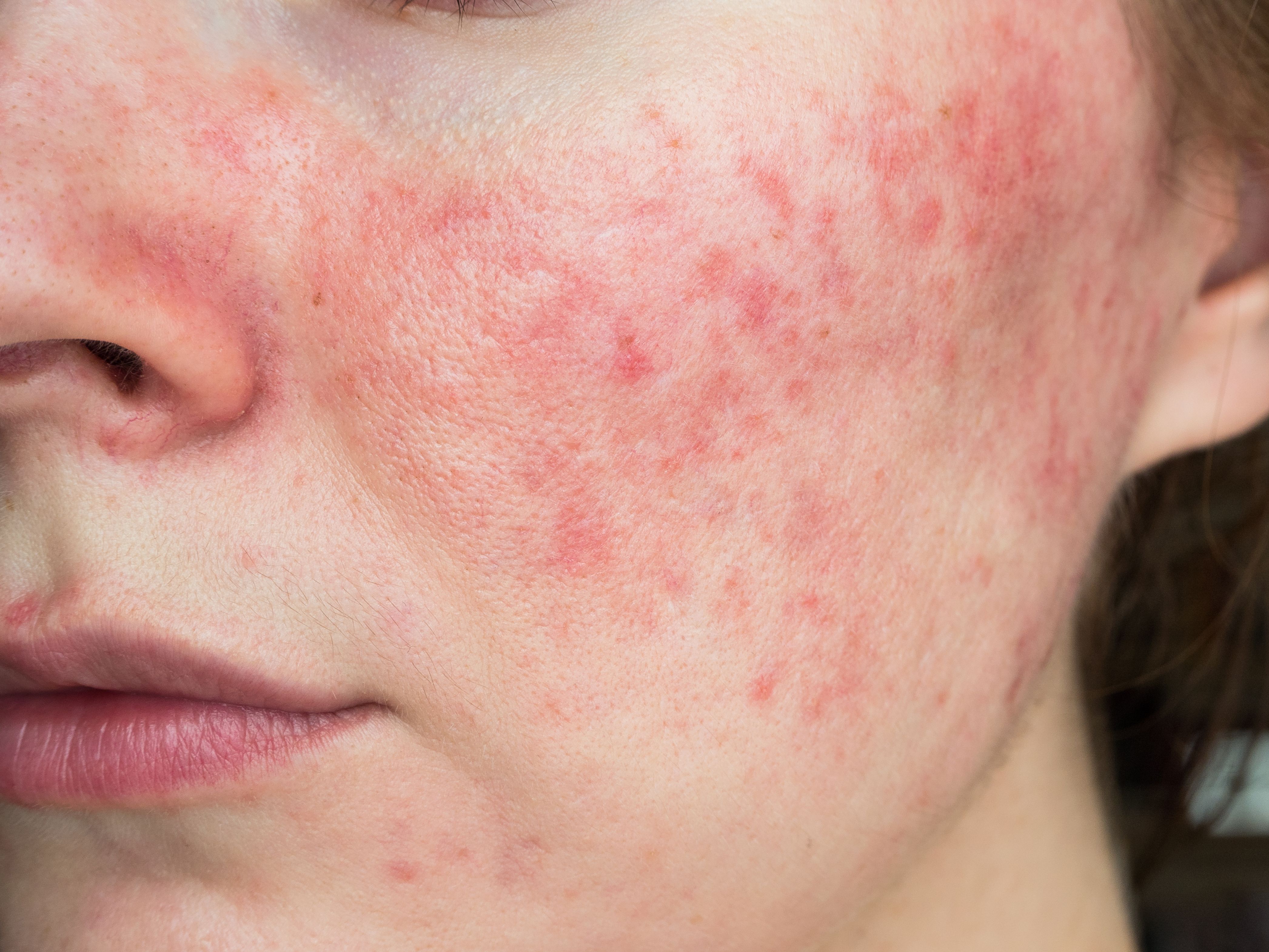
In Rosacea, Botulinum Toxin Requires Further Analysis of Long-Term Clinical Efficacy and Safety
Author(s):
While botulinum toxin has emerged as a treatment course for rosacea in recent years, researchers are calling for more comprehensive and detailed long-term research to assess its effectiveness.
While botulinum toxin has somewhat recentlyemerged as a potential therapeutic option for patients with rosacea, investigators involved in a recent systematic review and meta-analysis1 argue that the toxin lacks sufficient long-term clinical efficacy and safety.
Alessandro Grandini/AdobeStock

Investigators sought to evaluate the efficacy of botulinum toxin in rosacea in order to provide clinicians with a detailed and comprehensive analysis. They note that despite significant improvements in rosacea symptomology resulting from use of the emerging treatment, there is still a lack of clinical consensus surrounding long-term effects in this indication.
They began by collecting data related to botulinum toxin use in rosacea published up to May 25, 2023, using China Current Medical Content, the China National Knowledge Infrastructure, the Cochrane Central Register of Controlled Trials in the Cochrane Library, Embase, PubMed, the Wanfang database, and Web of Science. Papers were excluded for consideration if they lacked an accessible full text or possessed ambiguity, were animal experiments, or involved duplicate data. Guidelines, reviews, abstracts, notes, surveys, and non-peer reviewed dissertations were also ineligible for inclusion; meanwhile, randomized controlled clinical trials, non-randomized controlled trials, prospective or retrospective cohorts, case–control studies, case reports, and case series were all eligible for inclusion.
After the initial search process, 199 studies, trials, and reports were sent through a process of screening and cross-examination of data. Ultimately, 109 records were screened, 74 were assessed for eligibility, and 22 studies were included.
Represented forms of rosacea included erythematotelangiectatic rosacea, papulopustular rosacea, unspecified forms of rosacea, and flushing. All studies, however, involved the use of botulinum toxin A, with the vast majority (n=21) being delivered through intradermal injection. Some treatment routes were supplemented by combining therapeutic modalities, including use of intense pulsed light, a novel thermomechano-ablative device, pulsed dye laser, supramolecular salicylic acid and hyaluronic acid gel, and an ultrasound impact system.
All included studies reported efficacy in clinical improvements, reduced impact of disease, and overall patient and clinician satisfaction. Commonly-reported side effects of treatment included bruising or mild pain. Such effects were typically mild in nature and resolved spontaneously.
Furthermore, studies involving measure of Clinician's Erythema Assessment (CEA)demonstrated that patient CEA scores were significantly lower 1 month after treatment with botulinum toxin than at baseline with clinical significance in improved symptoms.
Limitations of the review and meta-analysis may include a risk of bias due to a limited number of relevant randomized controlled clinical trials, inconsistencies in follow-up visit duration, and a lack of studies directly comparing efficacy of treatment across varying skin types.
“Botulinum toxin is a novel option worth trying in terms of reduction in CEA score, improvement in signs and symptoms, and patient satisfaction. However, this conclusion is limited by the number and quality of research studies available,” according to He et al. “Larger samples and high-quality RCTs are needed to validate its long-term efficacy and recurrence rates to provide more credible evidence to clinicians. Additionally, there is an incomplete understanding of the prolonged adverse effects of botulinum toxin in patients requiring regular treatment. High costs can also discourage physicians and patients from using it as the preferred treatment modality.”
Reference
- He G, Yang Q, Wu J, et al. Treating rosacea with botulism toxin: Protocol for a systematic review and meta-analysis. J Cos Dermatol. 21 August 2023. https://doi.org/10.1111/jocd.15962