- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Imiquimod Capable of Detecting Subclinical Actinic Keratosis
Author(s):
As few as 90% of study participants without visible actinic keratosis lesions displayed inflammatory reactions indicative of the presence of subclinical AK lesions.
In as few as 90% of patients in a recent study,1 topical treatment with imiquimod revealed the presence of subclinical actinic keratosis (AK) despite clinical evidence of AK.
David A. Litman/Adobe Stock
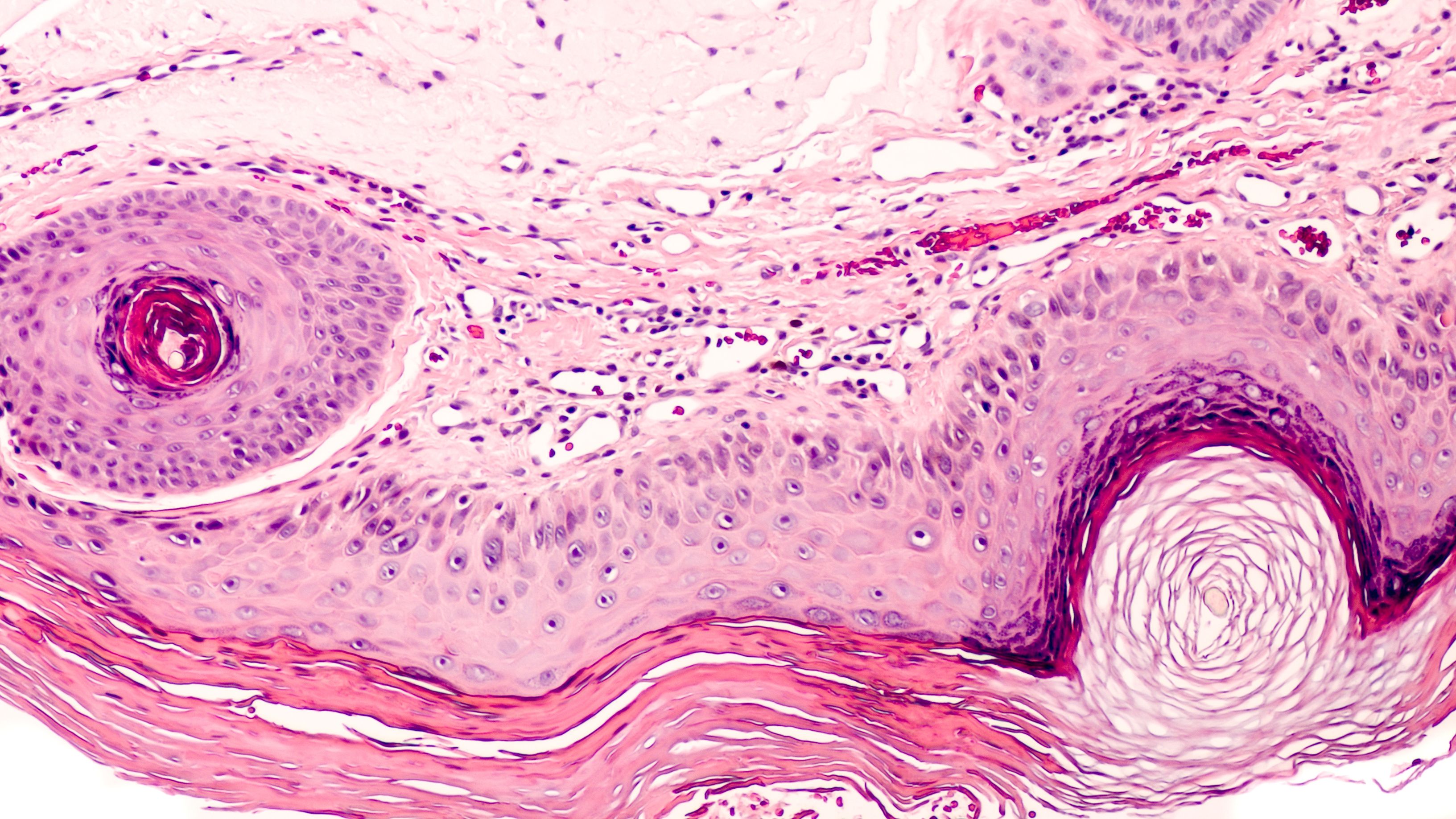
Investigators involved in the study, which was published in the Journal of the European Academy of Dermatology and Venereology, sought to clarify the existence of subclinical, nonvisible AK on skin with chronic ultraviolet (UV) exposure. During potential AK proliferation, lesions may represent invasive squamous cell carcinoma while remaining visibly undetectable. Furthermore, they hoped to differentiate differences in treatment outcomes between male and female patients.
Patient volunteers (n=46) between the ages of 50 and 82 years of age agreed to participate in the study. All patients had Fitzpatrick skin types 2 and 3 with clinical features of age dependent chronically UV-damaged skin. However, there were no clinically visible AK lesions present on the skin at the time of study inclusion.
At study baseline, all participants were photographed and completed a UV exposure questionnaire, including questions related to history of sunburn, hours spent outdoors, and more.
During the 2-week treatment period, all participants were asked to apply a topical imiquimod 3.75% cream to their faces on a nightly basis. Photography was repeated at the conclusion of the treatment period and 4 weeks later during follow-up.
On average, inflammatory responses started on day 5 of the treatment period. Approximately 90% of male participants demonstrated an inflammatory response to imiquimod that led to a demonstrable presence of subclinical AK lesions; meanwhile, this response led to the same outcome in 92% of female patients. Despite fewer male patients exhibiting this inflammatory response, on average, inflammation occurred 2 days earlier in male patients than in female patients.
Using a 4-point scale (0 to 3, with 3 indicative of greatest severity), investigators graded patients’ inflammatory responses by lesion size. On average, male and female patients displayed a difference in severity, with reaction severity lesser among female patients (n=1.4) versus male patients (n=2.6).
Adverse reactions to treatment included flu-like symptoms, aphthous ulcers, concomitant conjunctivitis, and swelling of the lips, all of which resolved by the time of the 4 week post-treatment follow-up. Despite lessened severity of reaction to imiquimod, female patients had a significantly higher rate of adverse reactions (n=58%) than their male counterparts (n=10%).
“The lower severity of cutaneous reaction in women than in men (overall severity value 1.4 in women vs. 2.5 in men) correlates with the fact that 58% of women in this study consistently use UV-protection daily compared with only 5% of men,” according to Kopera et al. “Overall, it is obvious that women in the 50+ generation have habitually used skincare products and UV-protection more frequently and consistently than men for decades, and due to this prophylaxis they appear to develop less subclinical AKs.”
Reference
- Kopera D, Torrano J, Soyer HP. Treating the invisible: Subclinical actinic keratosis detected by imiquimod. JEADV Clin Prac. Published online August 18, 2023. doi:10.1002/jvc2.216








