- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
Article
Hyaluronic acid filler for tear troughs lessens bruising, Tyndall effect risks
The first hyaluronic acid (HA) dermal filler indicated specifically for the tear troughs provides a safe, long-lasting alternative to previous HA options in this area, says Hassan Galadari, M.D.
The molecular structure of Teosyal Redensity 2 (HA, Teoxane), launched in the European Union in October 2012, provides the physical properties of a soft HA and the longevity of more durable fillers such as Radiesse (calcium hydroxylapatite/CaHA, Merz Aesthetics), says Dr. Galadari, assistant professor of dermatology, United Arab Emirates University, Dubai.
Tear troughs are a common problem worldwide, he says, “But nobody talks about them. Nobody tackles them, because there’s no really effective product in the United States.”
Tear troughs prove even more bothersome in the Middle East, because Fitzpatrick skin types 4 and 5 make them appear more noticeable, he explains. With darker skin types, “Any change in the tear troughs appears as dark circles under the eyes.”
Aging-related changes in the tear trough begin with atrophy of the cheek fat compartment. When this happens, “The tear troughs become even more noticeable,” Dr. Galadari says. “There are also some skeletal changes that occur.” Over time, bone resorption makes the orbital rim wider and the tear troughs more prominent.
Current challenges
The tear trough has proven difficult to treat partly because the skin in this area is extremely thin and translucent. Using popular HA fillers approved by the Food and Drug Administration can potentially lead to lumps or a bluish appearance under the skin in treated areas due to the Tyndall effect, Dr. Galadari says. This results from light reflecting off the clear HA gel.
Likewise, the vascularity of the tear trough area means that physicians must inject carefully or risk creating bleeders and bruising. “Additionally, cases of blindness have occurred due to embolisms in these vessels,” he says.
Further challenges stem from the fact that no FDA-approved fillers are indicated for the tear troughs, Dr. Galadari says. In the United States, “The current trend is to dilute any of the HA fillers with lidocaine to decrease the probability of the Tyndall effect. Products such as Juvéderm (Allergan) and Restylane (Medicis/Valeant) are mixed at a ratio of 1:1 or 2:1 for such an effect.” This practice makes the fillers easy to inject, he says.
Using such high concentrations of lidocaine biochemically alters the HA, however, and shortens its longevity, he says. No randomized, controlled trials have determined by how much, “But theoretically, it makes sense.” Adding water to any gel product breaks down its cohesion, just as water does to a lump of sugar, he explains.
“The key to HA’s longevity is its chemical makeup - the way that it’s cross-linked,” Dr. Galadari says. “If you break these cross-links with a lot of dilution, you end up with an HA that’s not going to last long.” Accordingly, he says, dermatologists in Europe and other international markets including the UAE don’t dilute HA products because they have many thinner HA fillers available.
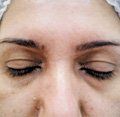
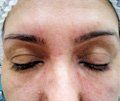
A tear trough before (left) and two weeks after correction using 0.5 mL of Teosyal Redensity 2 on each side in a 34-year-old female patient. The product was injected subdermally with a 28-gauge needle. (Photos: Hassan Galadar, M.D.)
Choice fillers
In the United States, the filler of choice for tear troughs is Belotero (HA, Merz), Dr. Galadari says.
“Some people dilute it with lidocaine, but you don’t have to. It’s a very thin product. It’s safe for injection into that area and provides satisfactory volume,” he says.
Other options for clinicians in the United States treating the tear trough include combining a hyaluronic acid such as Restylane with Radiesse in a manner first described by Lisa Donofrio, M.D. (68th Annual American Academy of Dermatology Meeting; March 5-10, 2010; Miami Beach, Fla.) “I’ve done this hundreds of times. It works fantastically,” Dr. Galadari says.
“The only dermal filler that has color is CaHA. But it’s a very thick product, so it should never be injected into the tear trough,” Dr. Galadari says. Rather, he says mixing a minute amount (0.1 mL) of CaHA into HA changes the HA’s color enough to avoid the Tyndall effect.
“The Restylane is treating the tear troughs, but because the Radiesse is there, there’s no chance of developing the Tyndall effect,” he says.
Dr. Galadari says he no longer uses the HA/CaHA combination, however, since Teosyal Redensity 2 became available.
“It’s composed of both cross-linked and non-cross-linked HA, in addition to amino acids and minerals. This creates a product that lasts for an average of a year. And no matter the quantity injected, it does not lead to any swelling or Tyndall effect,” Dr. Galadari says.
Because of its thin consistency, he says, Redensity 2 is virtually useless outside the tear troughs, its sole indication in Europe. It’s also available in Canada, but not the United States.
“Since June, I’ve injected at least 150 patients with Teosyal Redensity 2,” says Dr. Galadari, who was one of the product’s clinical investigators.
Regarding treatment technique, he says, “It’s injected the same way any other filler is injected,” with a needle or cannula. “It’s usually placed deeply, right on the inferior orbital rim. Then you massage it” to achieve the desired contour.
Relatively non-hygroscopic, Teosyal Redensity 2 will not absorb water from surrounding tissues, which is what produces swelling with other HA products, Dr. Galadari says. Because the product provides 1:1 correction, there’s no need to overcorrect. “Patients require a very small amount - usually 0.5 mL per side.”
Side effects
Regarding side effects, “There’s no swelling whatsoever,” Dr. Galadari says. Additionally, none of his patients have developed lumps. If patients somehow develop problems of this type, he notes that physicians can reverse the treatment with hyaluronidase.
As with other HAs, he says, injecting with a needle can produce bruising.
“Apart from that, there are no major drawbacks. Everybody I have injected has liked it, especially since it also contains lidocaine, making it a relatively pain-free procedure.” Many patients have referred family and friends for the treatment, he adds.
As for patient selection, Dr. Galadari says the product works well for virtually any patient concerned about tear troughs, except for those with more serious issues such as fat hypertrophy or significant eyelid sagging, both of which require blepharoplasty.
Because it’s such a specialized filler, Dr. Galadari says, Teosyal Redensity 2 costs patients a little less than Restylane - $400 versus $500 per treatment, respectively, in his market.
Overall, Redensity 2 exemplifies what Dr. Galadari sees as a trend toward increasing specialization in dermal fillers. In this regard, he says, Juvéderm Voluma (HA, Allergan) targets the cheeks and other areas requiring volume. He says he expects it to be available in the United States in early 2013.
Conversely, Restylane Lip Volume, available in the EU, targets the lips. “FDA clinical trials for that indication are ongoing in the United States,” Dr. Galadari says. One such trial was scheduled for completion in June 2012, according to www.clinicaltrials.gov. Medicis and Valeant representatives declined to comment on this study. Additionally, a phase 3 study is scheduled for completion in May 2013. DT
Disclosures: Dr. Galadari was an investigator for Teosyal Redensity 2 (Teoxane) and has received honoraria from Allergan, Q-Med (Restylane), Merz Aesthetics and Aqtis Medical. However, he reports no ongoing financial interests in these companies or products.





