- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
First Patient Dosed in IMG-007 Atopic Dermatitis Study
Author(s):
The OX40 monoclonal antibody possesses an extended half-life. In a previous study, this half-life exceeded that of the average conventional IgG.
The first patient has been dosed in a proof-of-concept study examining the efficacy, safety, and pharmacokinetics of Inmagene Biopharmaceuticals’ IMG-007, an OX40 receptor-binding humanized IgG1 monoclonal antibody (mAb), in adult patients with moderate to severe atopic dermatitis (AD).1
titikul_b/AdobeStock
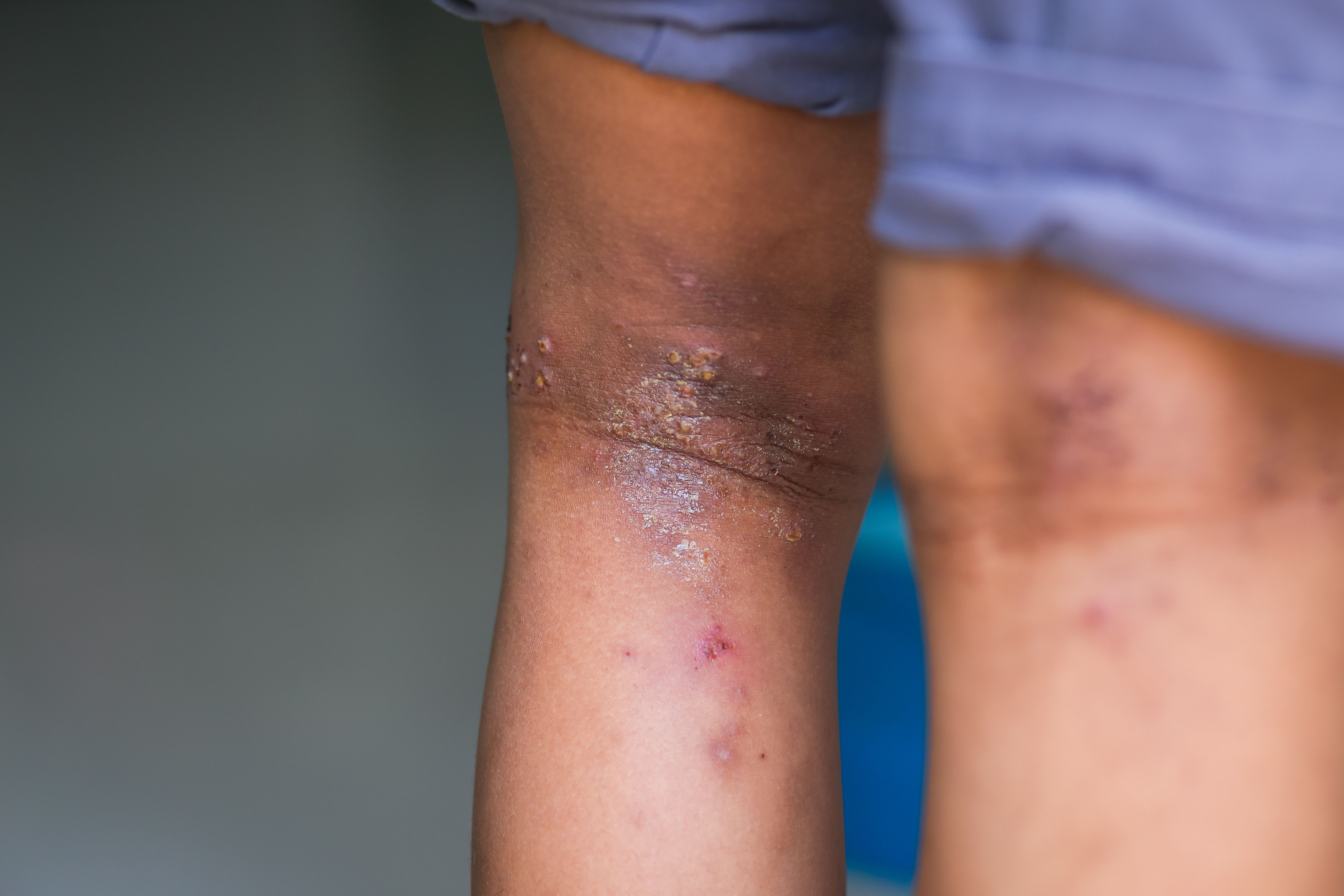
The phase 1b/2a, double-arm, open label study (NCT05984784) will examine the effects of IMG-007 in varying doses and consist of a 5-week screening period, a 12-week treatment period, and a 12-week follow-up period.2
Patients will be between the ages of 18 to 74 years of age with a moderate to severe AD diagnosis. Additionally, all participants must present with a documented history of inadequate response or lack of tolerability to 1 or more topical treatment options. Patients who are pregnant, lactating, or whohave an active infection, tuberculosis, pruritic skin condition, or a condition which may impact the safety of the treatment, are excluded from participation.2
Patients will be assigned via sequential assignment to 1 of 2 treatment arms, including a 300 mg or 600 mg dose of IMG-007, both to be administered intravenously 3 times over a 4-week period. Researchers will evaluate adverse events, pharmacokinetic characterization (via central compartment clearance and volume), and efficacy via the Eczema Area and Severity Index.2
At this time, it is estimated that the study will include 24 enrolled participants and conclude in May 2024.2
A previous study of the drug involving a single ascending dose in adult patients showed that IMG-007 possesses a half-life which exceeds that of the average conventional IgG. Target serum concentration was maintained from 12 to 18 weeks post-treatment, indicating the potential for extended “drug holidays,” and the drug was well-tolerated among study participants.2
"IMG-007 represents an investigational drug with multiple potential indications," said Yufang Lu, MD, PhD, and chief medical officer of Inmagene in a press release from the company.1 "We are excited to begin this trial of IMG-007 in AD patients, following favorable safety and highly differentiated pharmacokinetic results of the earlier trial. We are continuing our evaluations of IMG-007's potential in other diseases where OX40 signaling pathway plays an important pathogenic role."
References
- Inmagene. Inmagene’s OX40 MAB, with an extended half-life and silenced ADCC, enters POC study in atopic dermatitis. PR Newswire: press release distribution, targeting, monitoring and marketing. August 15, 2023. Accessed August 21, 2023. https://www.prnewswire.com/news-releases/inmagenes-ox40-mab-with-an-extended-half-life-and-silenced-adcc-enters-poc-study-in-atopic-dermatitis-301901200.html.
- CTG Labs - NCBI. Accessed August 21, 2023. https://www.clinicaltrials.gov/study/NCT05984784?term=inmagene+IMG-007&rank=1.






