- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
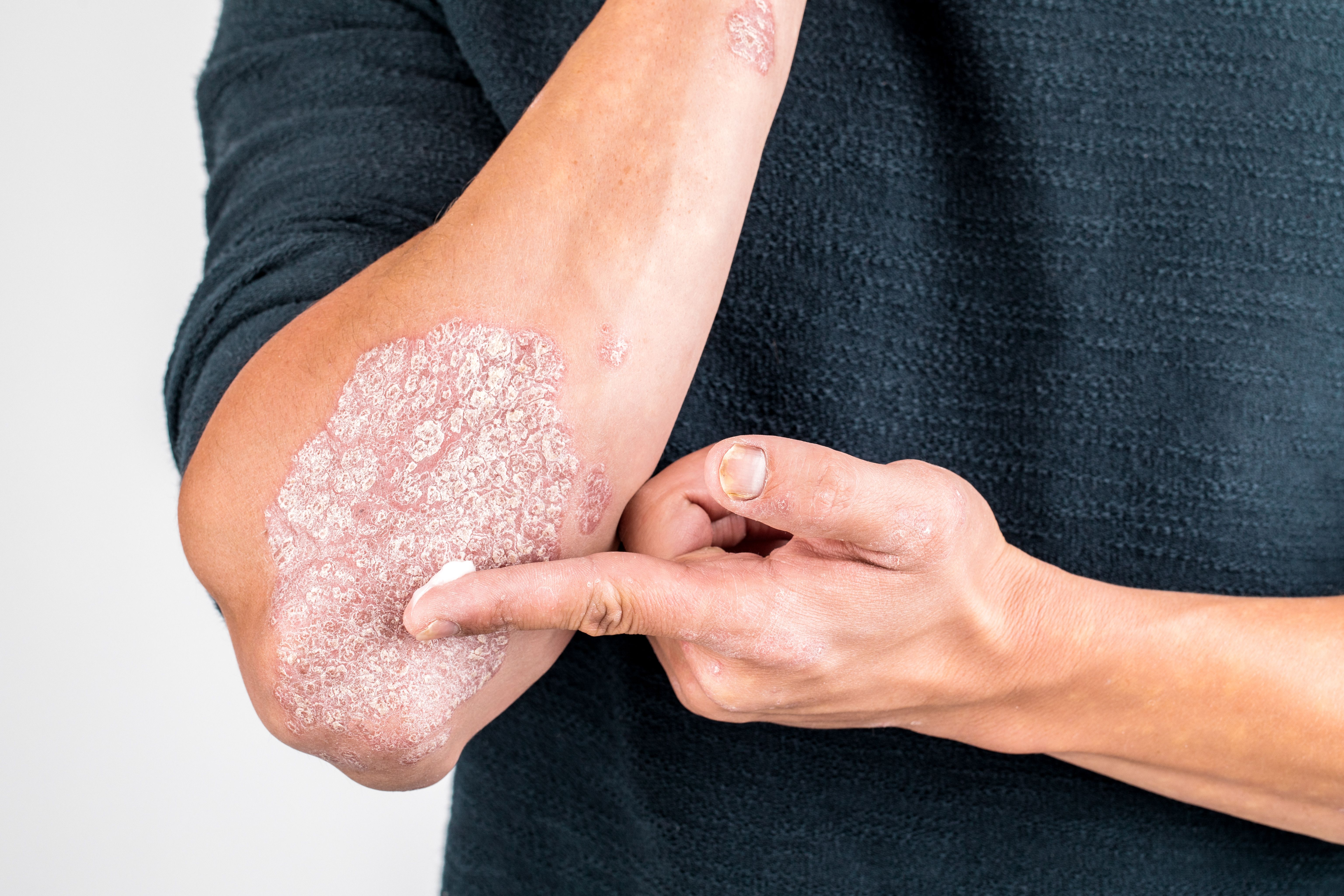
FDA clears new drug application for topical psoriasis treatment
The Food and Drug Administration accepted a new drug application for IDP-118 lotion (halobetasol propionate and tazarotene, Ortho Dermatologics), for the topical treatment of plaque psoriasis.
The Food and Drug Administration accepted a new drug application for IDP-118 lotion (halobetasol propionate and tazarotene, Ortho Dermatologics), for the topical treatment of plaque psoriasis.
The lotion, if approved, would be the first of its kind to combine tazarotene and halobetasol propionate in a single formulation for psoriasis in adult patients, according to the company, which is a division of Valeant.
In initial trials, the most common adverse events were application site pain (2.6 percent) and contact dermatitis (7.4 percent).