- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
FDA Clears Accure’s Laser System for Long-Term Treatment of Acne
Author(s):
Key Takeaways
- The Accure Laser System uses a 1726 nm wavelength to target sebaceous glands, offering a non-systemic acne treatment.
- Clinical trials demonstrated a 70% reduction in inflammatory lesions after four treatments, with consistent results across skin types.
This new indication adds to existing clearance of a non-drug treatment for mild to severe inflammatory acne vulgaris.
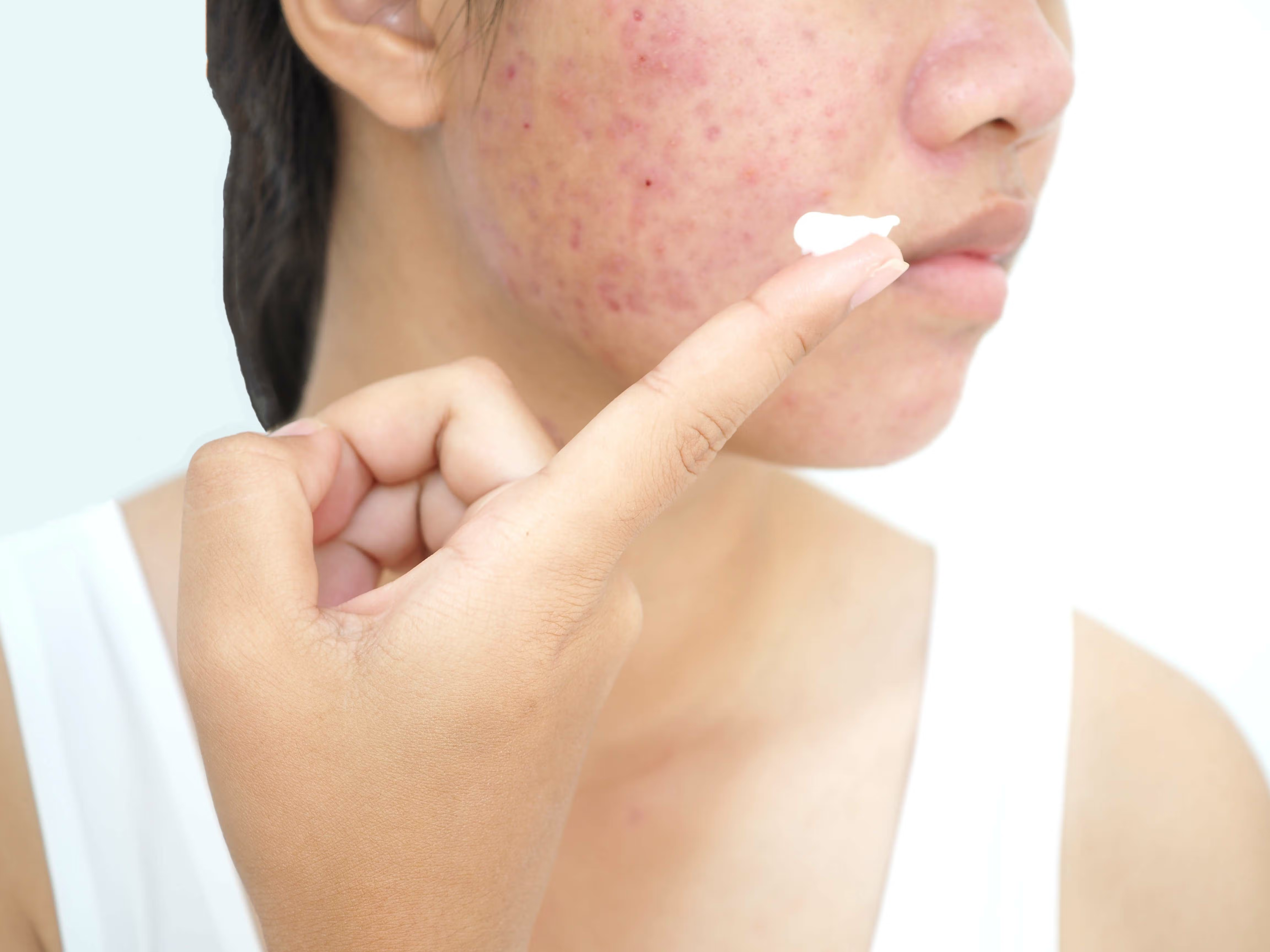
Image Credit: © dermnetnz.org

AccureAcne, Inc. announced today that the US Food and Drug Administration (FDA) has cleared its Accure Laser System for long-term treatment.1 The laser system was first approved in 2022 for treating mild to severe inflammatory acne vulgaris, becoming the first laser platform to do so.2
The success of the Accure Laser System is based on its unique 1726 nm laser wavelength, adding proprietary technology to carefully control thermal gradient depth and target the sebaceous gland. Emil Tanghetti, MD, founder of The Center for Dermatology and Laser Surgery and the first Accure Laser investigator, stated that the use of forced air cooling and real-time monitoring algorithms is what separates this method from more traditional options.
"This accomplishment is a testament to the relentless pursuit of this goal by Accure, Quanta System, and the team of clinicians and researchers who have spent many years together to bring the Accure Laser to the world,” said Tanghetti. "Our journey in understanding the unique nature of this wavelength and the clinical requirements needed to deliver significant clinical outcomes was certainly challenging.”1
Clearance for long-term treatment was supported by multiple US clinical trials approved by Institutional Review Boards. After a series of 4 treatments, the average inflammatory lesion count reduced by 70% at the 6-month mark. These results were consistent among all skin types and severity levels of acne.
About 50% of patients are unsatisfied with the current treatment options for acne vulgaris.3 According to Accure, the targeted, non-systemic treatment provides an average of 70% acne reduction after the 4 treatments without the side effects commonly associated with prescription medications. Up to 95% of patients were very or extremely satisfied with the results.
Accure Acne’s limited commercial release in the US earlier this year demonstrated strong clinical outcomes and high satisfaction levels among both patients and providers. These satisfaction scores averaged over 4.4 out of 5. Additionally, the company expanded the accessibility of its laser system to regions such as the Middle East, Europe, The Caribbean, and Asia Pacific.
"This achievement cannot be overstated," Christopher Carlton, Accure’s co-founder, chairman of the board, and chief executive officer, said in a statement. "After many years of rigorous technical, scientific, and clinical development, The Accure Laser has delivered yet another milestone achievement-the ability to provide a significant, sustained, and durable drug-free alternative for acne patients and their providers."1
References
1. Accure acne announces new FDA clearance for the long-term treatment of acne. Accure Acne, Inc. Published October 15, 2024.https://www.prnewswire.com/apac/news-releases/accure-acne-announces-new-fda-clearance-for-the-long-term-treatment-of-acne-302276293.html.
2. FDA clears the Accure laser system for the treatment of mild to severe inflammatory acne vulgaris. Accure Acne, Inc. Published November 22, 2022.https://accureacne.com/fda-clears-accure-laser-system-treatment-mild-severe-inflammatory-acne-vulgaris
3. Hayran Y, İncel Uysal P, Öktem A, Aksoy GG, Akdoğan N, Yalçın B. Factors affecting adherence and patient satisfaction with treatment: a cross-sectional study of 500 patients with acne vulgaris. J Dermatolog Treat. 2021;32(1):64-69. doi:10.1080/09546634.2019.1618434