- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
FDA Approves Ustekinumab-auub as Interchangeable Biosimilar to Stelara for Plaque Psoriasis, Psoriatic Arthritis, and More
Author(s):
The approved indication makes Wezlana the first approved interchangeable biosimilar to Stelara.
pimentos/Adobe Stock
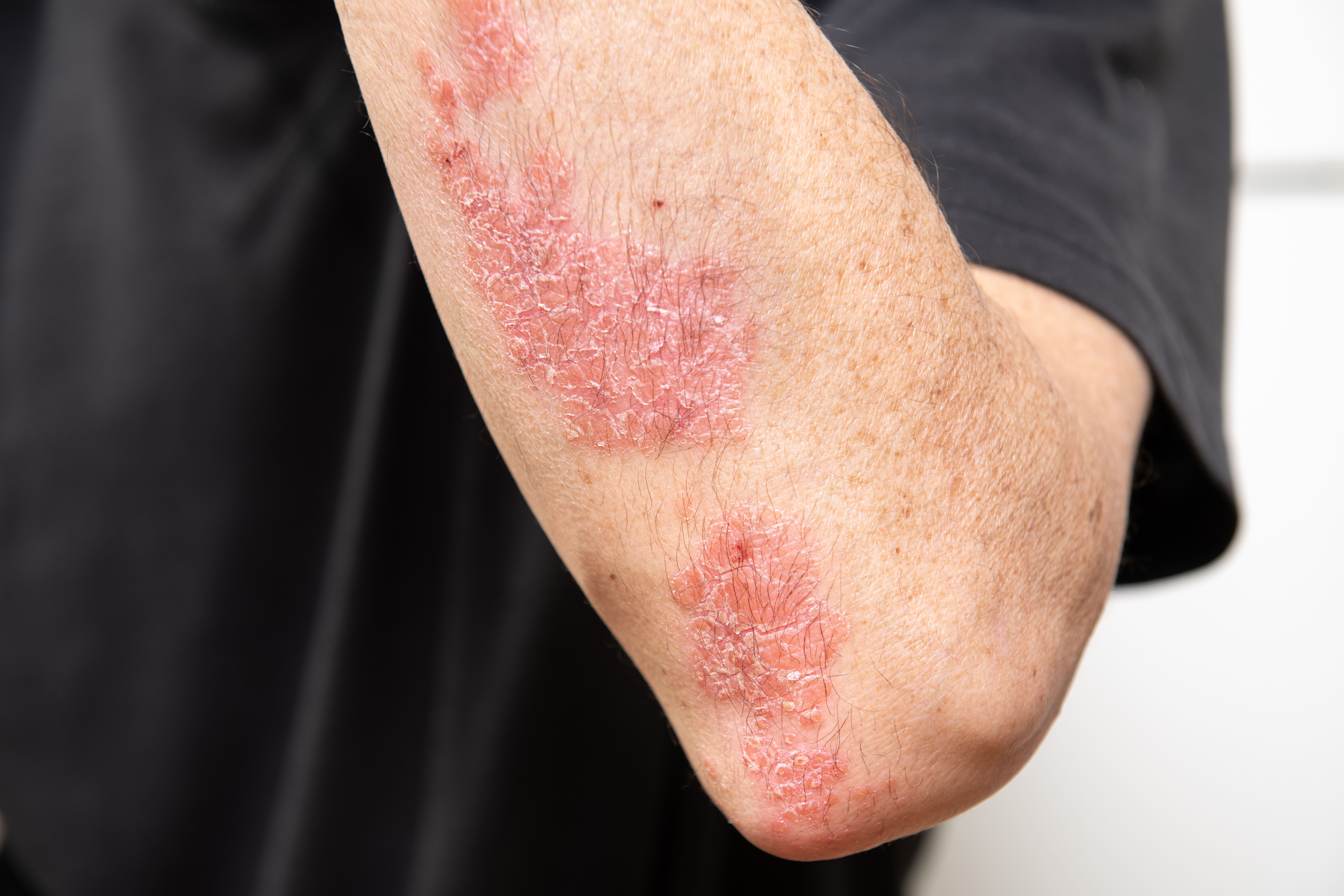
Ustekinumab-auub (Wezlana), an interchangeable biosimilar to ustekinumab (Stelara) has been approved by the FDA for use in multiple inflammatory diseases, according to an FDA press release.1
Like its reference product, ustekinumab-auub is approved for use in adult patients with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy, with active psoriatic arthritis, with moderately to severely active Crohn disease, and with. Moderately to severely active ulcerative colitis. For pediatric populations, the drug is approved for use in those with moderate to severe plaque psoriasis who are candidates for phototherapy or systemic therapy and those with active psoriatic arthritis.
“Biosimilar medications offer additional safe and effective treatment options that have the potential to increase access for people requiring treatment for inflammatory diseases,” said Nikolay Nikolov, MD, director of the Office of Immunology and Inflammation at the Center for Drug Evaluation and Research.1 “Today’s approval could have a meaningful impact for patients managing their disease.”
The approval of ustekinumab-auub was based on a review of evidence demonstrating that the drug is highly similar to the reference product, with no clinically meaningful differences in terms of safety, purity, potency, or effectiveness. Data included product comparisons “on an analytical level using an extensive battery of chemical and biological tests and biological assays” which confirmed structural and functional similarities. Human pharmacokinetic, clinical immunogenicity, and other clinical safety and effectiveness data were also reviewed.
Additionally, this evidence demonstrated that ustekinumab-auub met legal requirements to be interchangeable with ustekinumab at the pharmacy level.
Similar to the reference product, the most serious side effect of ustekinumab-auub is infection; the most common adverse reactions include nasopharyngitis, upper respiratory tract infection, headache, fatigue, nausea, vomiting, and injection site erythema, among other reactions. Labeling includes a warning about an increased risk of serious infections leading to hospitalization, as well as a warning that malignancies, hypersensitivity reactions, and cases of Posterior Reversible Encephalopathy Syndrome were reported in clinical studies.
By 2025, 2 additional ustekinumab biosimilars are expected to launch.2 AVT04, manufactured by Alvotech and Teva, is anticipated by February 21, 2025, following a complete response letter due to a deficiency in an Alvotech facility in Iceland. The second, FYB202, manufactured by Fresenius Kabi and Formycon, is anticipated to launch no later than April 15, 2025.
References
- FDA approves interchangeable biosimilar for multiple inflammatory diseases. News release. FDA. October 31, 2023. Accessed November 1, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-interchangeable-biosimilar-multiple-inflammatory-diseases
- FDA approves first interchangeable Stelara biosimilar. Formulary Watch. November 1, 2023. Accessed November 1, 2023. https://www.formularywatch.com/view/fda-approves-first-interchangeable-stelara-biosimilar
[This article was originally published by our sister publication, Drug Topics.]





