- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Examining Rates of Conjunctivitis in Dupilumab-Treated Patients
Author(s):
A late breaking session from Revolutionizing Atopic Dermatitis Virtual Conference analyzed the frequency and clinical meaningfulness of conjunctivitis.
Alessandro Grandini/Adobe Stock
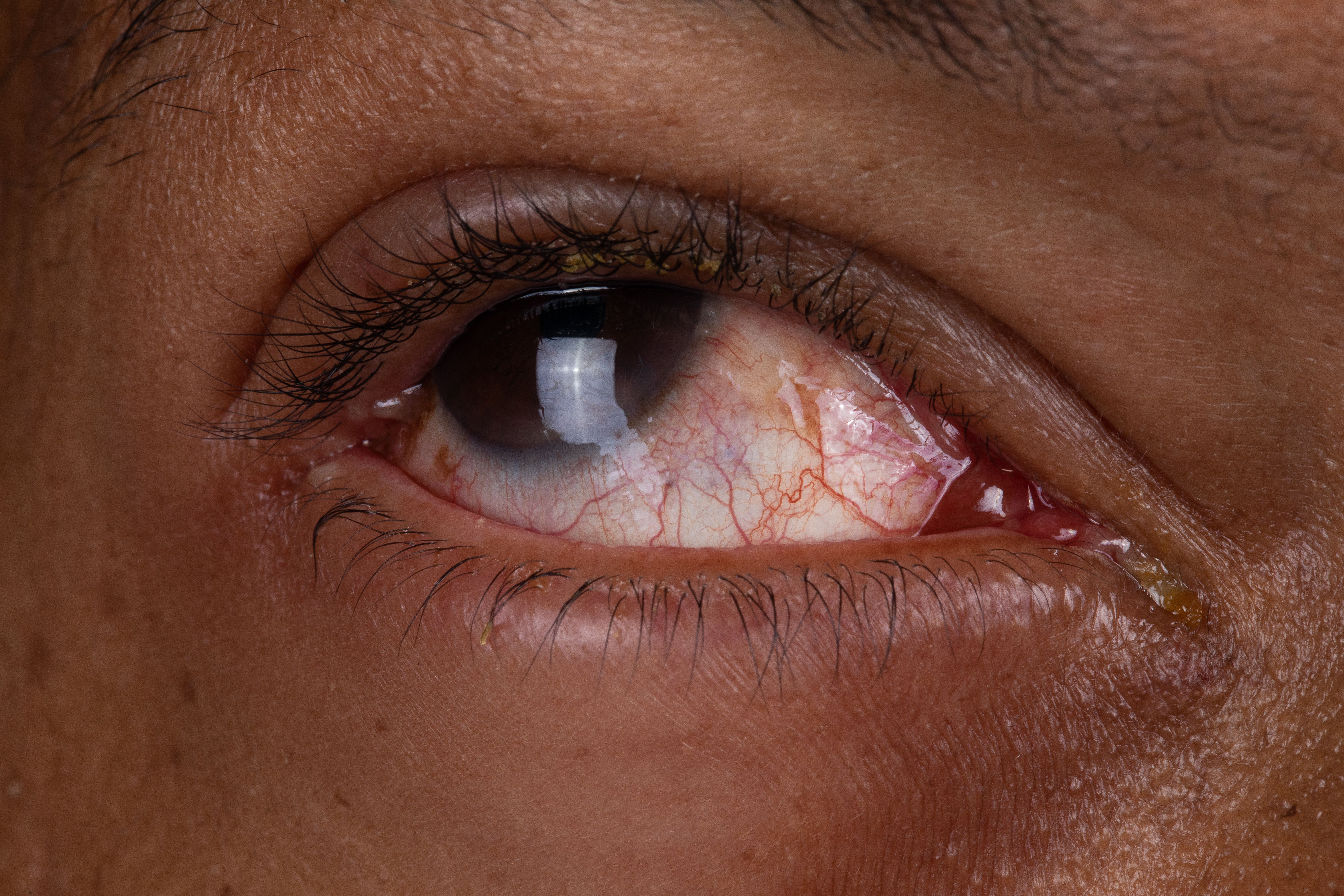
Dupilumab, known for inhibiting both IL-4 and IL-13, has been associated with conjunctivitis as a comorbidity in atopic dermatitis (AD). In a recent late-breaking presentation from the 2023 Revolutionizing Atopic Dermatitis Virtual Conference, Matthews Zirwas, MD, delved into the topic of dupilumab-induced conjunctivitis.
The presentation aimed to address lingering questions surrounding the frequency and clinical significance of this adverse event, particularly among patients with AD.
"An interesting part of what we need to keep in mind is that conjunctivitis is just in comorbidity for atopic dermatitis in general, and that's where this gets really interesting," Zirwas said. "How do we differentiate the cases that are dupilumab-induced versus the cases that are just part of the underlying disease process?"
Zirwas detailed a study in which researchers assessed a series of randomized, double-blinded, and placebo-controlled trials conducted under regulatory conditions. The studies encompassed 70 trials in varying age groups, including adults, adolescents, school-aged children, and preschoolers. The trials varied in duration, ranging from 16 to 52 weeks in length.
Analyzing raw data, Zirwas addressed misconceptions around post-marketing studies reporting conjunctivitis rates as high as 20%.
By dissecting the 16-week monotherapy trials, the findings revealed a significant disparity between placebo (2%) and dupilumab-treated (9.3%) conjunctivitis cases.
"We cannot say that Dupixent causes conjunctivitis in 20% of patients, because we know that the vast majority of conjunctivitis cases happen in the first 16 weeks," he said.
Expanding the scope to 52-week trials, Zirwas observed a marginal increase in conjunctivitis cases, emphasizing that the majority of clinically meaningful cases likely occur early in the treatment.
Researchers noted that persistent conjunctivitis cases that endured throughout the trials occasionally led to participant dropout. Approximately 2% of patients treated with dupilumab experienced clinically meaningful conjunctivitis, potentially therapy-limiting. This pattern persisted across age groups and trial durations.
"This is just not something we see in people treated with dupilumab for other indications," Zirwas said.
Reference
Zirwas M. Conjunctivitis adverse events in dupilumab clinical trials. Late-breaking research presented at: Revolutionizing Atopic Dermatitis Virtual Conference, December 10, 2023.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









