- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Dupilumab for Atopic Dermatitis: 7 Years Later
Author(s):
Today officially marks 7 years since the US FDA approved dupilumab for the treatment of atopic dermatitis. Here's a look back at its approval timeline and all it has accomplished in the dermatology world in the time since.
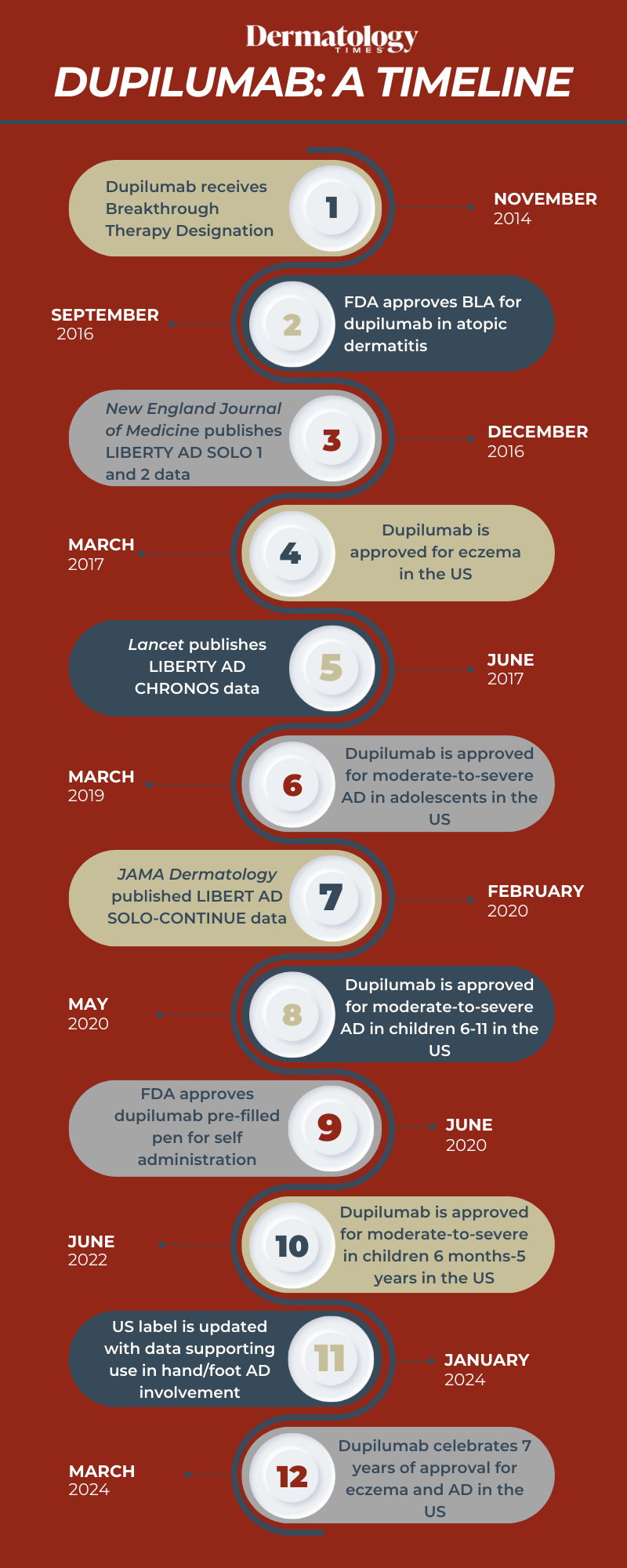
The US Food and Drug Administration (FDA) first approved Regeneron and Sanofi's dupilumab (Dupixent) for patients with atopic dermatitis (AD) in March 2017.1 In the time since, dupilumab has become a gold-standard treatment for patients with the condition, including adults, adolescents, young children, and infants.
Today now marks 7 years to the date that dupilumab received this approval. Dermatology Times reflects on the timeline of dupilumab over the years, including strides and approvals made during this time.
November 2014: Dupilumab receives Breakthrough Therapy designation from US FDA
Regeneron Pharmaceuticals, Inc. and Sanofi received Breakthrough Therapy designation from the FDA for dupilumab, intended for treating moderate-to-severe AD in adults not adequately controlled by current therapies.2
The designation aimed to expedite the drug's development and review process due to the limited treatment options for this condition. Based on promising phase 1 and 2 trial results, the designation is reserved for drugs addressing serious or life-threatening conditions and requires evidence of significant improvement over existing therapies or placebo. At the time, a phase 3 clinical program for dupilumab was underway worldwide.
September 2016: FDA accepts BLA for dupilumab in atopic dermatitis
Sanofi and Regeneron Pharmaceuticals announced that the FDA accepted the Biologics License Application (BLA) for dupilumab for priority review.3
At the time, the application had a target action date of March 29, 2017. It included data from 3 phase 3 studies involving over 2,500 patients globally. The studies assessed dupilumab as monotherapy and in combination with topical corticosteroids for patients whose disease was not adequately controlled by existing therapies.
March 2017: Dupilumab is approved for eczema in the US
The US FDA approved dupilumab injection to treat adults with moderate-to-severe eczema.1 Dupilumab was intended for patients whose eczema was not adequately controlled by topical therapies, or those for whom topical therapies were not advisable. It could be used with or without topical corticosteroids.
The safety and efficacy of dupilumab were established in 3 placebo-controlled clinical trials with a total of 2,119 adult participants with moderate-to-severe AD not adequately controlled by topical medication(s). Overall, participants who received dupilumab achieved a greater response, defined as clear or almost clear skin, and experienced a reduction in itch after 16 weeks of treatment.
March 2019: Dupilumab is approved for moderate-to-severe AD in adolescents in the US
Regeneron and Sanofi announced that the US FDA approved dupilumab for adolescent patients aged 12 to 17 with moderate-to-severe AD whose disease wasn't adequately controlled with topical prescription therapies or when those therapies weren't advisable.4 Dupilumab could be used with or without topical corticosteroids.
In the pivotal phase 3 trial evaluating dupilumab monotherapy in adolescent patients with uncontrolled moderate-to-severe AD, the safety and efficacy were generally consistent with that previously seen in adult studies. At 16 weeks:
- The average improvement in the Eczema Area and Severity Index (EASI) from baseline was approximately 66% compared to 24% for placebo.
- More than 10 times as many patients had clear or almost clear skin with dupilumab compared to placebo.
- Over 5 times as many patients saw overall disease improvement of at least 75% with dupilumab compared to placebo.
- Over 7 times as many patients experienced significantly reduced itch with dupilumab compared to placebo.
Dupilumab had been studied in more than 7,000 patients aged 12 years and older in over 30 clinical trials. Its safety profile in the adolescent trial was similar to the safety profile from trials in adults with atopic dermatitis and consistent through 52 weeks. The most common adverse events were injection site reactions, eye and eyelid inflammation including redness, swelling and itching, pain in the throat (oropharyngeal pain) and cold sores in the mouth or on the lips.
May 2020: Dupilumab is approved for moderate-to-severe AD in children 6-11 in the US
The US FDA approved dupilumab for children aged 6 to 11 years with moderate-to-severe AD whose disease wasn't adequately controlled with topical prescription therapies or when those therapies weren't advisable.5 Dupilumab became the only biologic medicine approved for this patient population.
The FDA approval was based on data that included pivotal phase 3 results on the efficacy and safety of dupilumab combined with topical corticosteroids (TCS) compared to TCS alone in children with severe AD. In the trial, children treated with dupilumab and TCS experienced significant improvements in overall disease severity, skin clearance, and itch.
June 2022: Dupilumab is approved for moderate-to-severe AD in children 6 months-5 years in the US
In June 2022, the US FDA approved dupilumab as the first biologic medicine for children aged 6 months to 5 years with moderate-to-severe AD.6 Dupilumab became the sole biologic medicine authorized for treating moderate-to-severe AD from infancy to adulthood.
Children treated with dupilumab alongside TCS attained clearer skin and experienced significantly reduced itch compared to those treated with TCS alone in a phase 3 trial conducted over 16 weeks. Long-term safety data from a 52-week open-label extension trial in this age group reinforced the well-established safety profile of dupilumab observed across all other approved age groups.
January 2024: US label is updated with data supporting use in hand/foot AD involvement
The US FDA updated the label for dupilumab in AD, incorporating efficacy and safety data for patients aged 12 years and older with AD with uncontrolled moderate-to-severe hand and/or foot involvement.7 These phase 3 data originated from the first and only trial evaluating a biologic specifically for this challenging-to-treat population, and they were also added to the dupiluman label in the European Union, with additional regulatory submissions in progress.
Data from the Phase 3 LIBERTY-AD-HAFT trial showed that more than twice as many patients treated with dupilumab achieved clear or almost clear skin on hands and feet, and nearly 4 times as many experienced improvement in itch compared to those on placebo. At 16 weeks, significant improvements were observed in both clear skin and reduction in itch for patients treated with dupilumab.
References
- FDA approves Dupixent. Drugs.com. March 28, 2017. Accessed March 27, 2024. https://www.drugs.com/newdrugs/fda-approves-dupixent-dupilumab-eczema-4505.html
- Regeneron and Sanofi announce that dupilumab has received FDA Breakthrough Therapy designation in atopic dermatitis. News release. Regeneron. November 20, 2014. Accessed March 27, 2024. https://investor.regeneron.com/news-releases/news-release-details/regeneron-and-sanofi-announce-dupilumab-has-received-fda
- Sanofi and Regeneron announce dupilumab Biologics License Application accepted for priority review by US FDA. Drugs.com. September 26, 2016. Accessed March 27, 2024. https://www.drugs.com/nda/dupixent_160926.html
- FDA approved Dupixent (dupilumab) for moderate-to-severe atopic dermatitis in adolescents. News release. Regeneron. March 11, 2019. Accessed March 27, 2024. https://investor.regeneron.com/news-releases/news-release-details/fda-approves-dupixentr-dupilumab-moderate-severe-atopic#:~:text=(NASDAQ%3A%20REGN)%20and%20Sanofi,therapies%20or%20when%20those%20therapies
- FDA approves Dupixent (dupilumab) as first biologic medicine for children aged 6 to 11 years with moderate-to-severe atopic dermatitis. News release. Sanofi. May 26, 2020. Accessed March 27, 2024. https://www.sanofi.com/en/media-room/press-releases/2020/2020-05-26-15-40-00-2038798
- FDA approves Dupixent (dupilumab) as first biologic medicine for children aged 6 months to 5 years with moderate-to-severe atopic dermatitis. News release. Sanofi. June 7, 2022. Accessed March 27, 2024. https://www.sanofi.com/en/media-room/press-releases/2022/2022-06-07-20-45-00-2458243
- Dupixent (dupilumab) US label updated with data further supporting use in atopic dermatitis with moderate-to-severe hand and foot involvement. News release. Sanofi. January 16, 2024. Accessed March 27, 2024. https://www.sanofi.com/en/media-room/press-releases/2024/2024-01-16-12-00-00-2809681






