- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Deucravacitinib: A Year in Review
Author(s):
As deucravacitinib surpasses its 1-year milestone since approval, clinicians continue to monitor its real-world performance among patients.

The FDA approved deucravacitinib (Sotyktu; Bristol Myers Squibb) for the treatment of adults with moderate to severe plaque psoriasis in September 2022.1 Since its approval, deucravacitinib has become a necessary addition to the psoriasis armamentarium for dermatology clinicians. The allosteric TYK2 inhibitor provides patients with an oral treatment option, further helping those who may not tolerate or achieve clear skin while using topical therapies.
Deucravacitinib’s groundbreaking approval was based on data from the phase 3 POETYK-PSO-1 (NCT03624127) and POETYK-PSO-2 (NCT03611751) clinical trials. Its new mechanism of action was proven efficacious compared with placebo and twice-daily apremilast (Otezla; Amgen).1
As deucravacitinib reaches its 1-year milestone since approval, clinicians continue to monitor deucravacitinib’s real-world performance among patients and consider how it will further establish itself among available psoriasis therapies. To further discuss the importance of deucravacitinib’s approval and what truly defines success, Dermatology Times spoke with experts in psoriasis management to provide additional insights.
Q: How has efficacy been maintained since the initial POETYK phase 3 trial, as well as 1 year out since deucravacitinib’s approval?
Bhatia: Since the launch of deucravacitinib, I personally have seen the vast majority of patients maintain clearance after the initial month, similar to what was observed in the trials. Our site still has a few [participants] in the long-term extension trial, and they do not want to drop out [because] they have not experienced clearance with an oral therapy like this in the past. Overall, there are small areas of breakthrough that can happen with missed doses, but they are mitigated with a topical agent that the patients can use at their discretion.
Gooderham: The patients I have treated on commercial medication have been able to maintain the efficacy reached by about month 4 to 6, the latest time point since starting. I had patients in the clinical trials who have been able to maintain their efficacy for about 3 years.
Miller: The efficacy of deucravacitinib in plaque psoriasis was maintained to 52 weeks and demonstrated superiority, achieving PASI 75, PASI 90, and sPGA 0/1 in deucravacitinib vs both placebo and apremilast, the active comparator in the pivotal trials. Likewise, in POETYK PSO-LTE [NCT04036435], patients who were on deucravacitinib continuously for 2 years maintained PASI 75 response in a post hoc subanalysis. This long-term durability is critical in chronic inflammatory conditions such as plaque psoriasis.
Clinically, I have seen similar efficacy as was seen in the POETYK trials. I have seen similar efficacy both short term at the primary end point at 16 weeks and long term out to 52 weeks. I have not seen a waning of response thus far. I am optimistic my patients will maintain their efficacy consistent with [findings from] the long-term extension study.
Q: What, if any, adverse events have you observed in your patients? How were they managed?
Bhatia: I have not seen anything that is worth reporting because either these patients do not come back for a year after we start them or they are managing themselves with the combination of a topical treatment for spots. These are often patients who do not want biologics or want a change to an oral therapy, plus the ease of compliance and safety without regular laboratory monitoring keeps the patients on board.
Gooderham: I did have one patient with mouth ulcers, which resolved with time, and one patient had herpes zoster in the first few months of therapy but was treated with an antiviral medication with no residual effects. Most of the patients do not report any tolerability issues at all.
Miller: I have had a handful of patients experience acneiform eruptions. I have been able to manage the acneiform eruptions with standard topical therapy, with resolution.
Q: What makes deucravacitinib different from other available psoriasis therapies, such as biologics, topicals, or oral medications?
Bhatia: Simply put, it is different because it is safe and works.
Gooderham: Deucravacitinib is different because it is easy to take: 1 pill, once a day, no laboratory monitoring, and few tolerability issues and potential adverse effects. It’s easy to travel with and can be incorporated conveniently into a daily routine. There are patients who do not want another cream and others who are reluctant to start injections, so a once-a-day pill appeals to them.
Miller: Deucravacitinib stands out among other psoriasis therapy options due to its unique mechanism of action. Unlike traditional treatments, it offers a more targeted approach. Deucravacitinib is an oral selective allosteric tyrosine kinase inhibitor (TYK2), a novel new-in-class therapy. It inhibits TYK2 via an allosteric mechanism by selectively binding to the unique regulatory domain rather than the active catalytic domain of the enzyme like other Janus kinases. It prevents activation of TYK2 and inhibits downstream signaling of IL-23, IL-12, and type 1 IFN which are implicated in a number of autoimmune diseases.
When compared with other systemic therapy, specifically its closest competitor, apremilast, deucravacitinib offers convenient, once-daily dosing and tolerability. Compared with topical therapies, deucravacitinib’s systemic nature allows it to address widespread inflammation.
Q: Has deucravacitinib’s safety and efficacy been adequately tested in patients with skin of color?
Bhatia: The study demographics included many [participants] with darker skin types and the response rates and safety data were in line with those reported in the entire study.
Gooderham: The majority of patients in the POETYK study were white; however, in POETYK- PSO-1, as many as 20% of patients were Asian. There was very low enrollment of Black study participants (less than 2% to 4%), so there is still more to learn about the safety and efficacy of deucravacitinib across the entire skin tone spectrum.
Miller: Disparities in patients with skin of color are evident in psoriasis clinical trials as these trials often underrepresent diverse populations. This often leads to gaps in understanding treatment efficacy and safety across different skin types.
Only 2% of patients in deucravacitinib clinical trials had skin of color. However, when looking at this group, the efficacy and safety were the same as the entire trial population.
Q: What considerations are different when evaluating psoriasis in patients with skin of color?
Bhatia: The most important assessment is erythema and the ability of the investigator or dermatologist to identify postinflammatory vs active violaceous erythema in darker skin. To many patients with darker skin types, the residual presence of dusky violaceous change may indicate persistence of disease, whereas in lighter skin the residual light pink to red changes are often a sign of improvement or near resolution.
Gooderham: There are many considerations in patients with skin of color, such as some challenges with PASI scoring, as erythema may not be easily assessed, and this needs to be taken into consideration. Also, patients may present with more severe disease due to delays in diagnosis and less use of advanced therapies. Access can be an issue, and I hope this is addressed in some way, such as a specific trial in patients with skin of color or an access program. I also look forward to real-world evidence, perhaps from CorEvitas, that may have more diversity in patients enrolled.
Miller: Gauging the severity of lesions in patients with skin of color is known to be a difficult process. Providers need a nuanced approach when evaluating different skin tones. In patients with skin of color, erythema can appear violaceous or grey and can lead to a misdiagnosis or missed diagnosis. It can be tricky to differentiate between erythema and postinflammatory hyperpigmentation in darker skin types. Depending on the skin type, location and severity of disease can differ as well. As a provider, I must keep in mind presentation, treatment response, and potential cultural implications to provide effective care.
Q: What real-world opinions and outcomes do you see and hear from your patients about their journey on deucravacitinib?
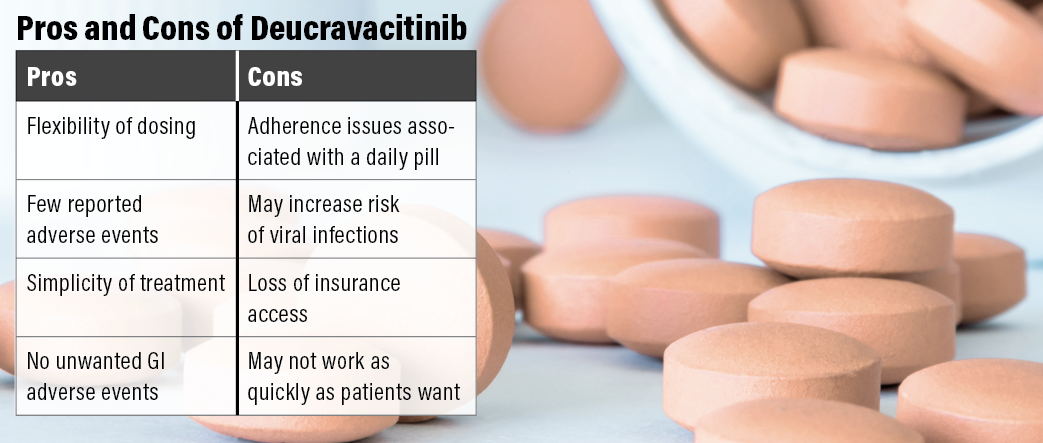
Bhatia: The pros are the flexibility of dosing and safety, with very few reported adverse effects, and ease of taking the tablet. The con is the need to remember to take the dose.
Gooderham: The pros of deucravacitinib in the real world that I hear from my patients are convenience and simplicity of treatment; it is better tolerated than previous oral therapies they have tried such as methotrexate, apremilast, and acitretin; and it has easier accessibility than a biologic. Some cons include that it doesn’t work the same for everyone (some home runs, but some patients don’t respond as well), an increased risk of viral infection (patient with herpes zoster), ongoing therapy is required, and sometimes patients lose access or insurance changes, etc.
Miller: Overall, my patients have been happy with this new therapy option. Many of my patients were taking apremilast and experienced unwanted GI adverse effects. They have not with deucravacitinib. Having another therapy option that allowed them the convenience of oral administration but without those troublesome GI issues has been greatly appreciated.
The con I’ve heard from patients is that deucravacitinib isn’t working as quickly as they might like. But some of these patients have a history of using biologics. I set expectations with them prior to initiating therapy. However, their disease significantly impacts their quality of life. They want quick improvement in their appearance and symptoms. Biologics can offer a quicker onset of action, but some patients may not be candidates or are needle-phobic. Those patients who choose systemic therapy must be willing to sacrifice a slightly slower onset of action for the convenience of an oral therapy.
Q: What is important for you as a clinician to see in the continued success of deucravacitinib?
Bhatia: We are all grateful to Bristol Myers Squibb for the support of the drug as well as for promoting access through specialty pharmacies and enrollment programs.
Gooderham: I would like to see some real-world data, particularly in a wider range of patients, including across the skin tone spectrum. I hope there are some access programs for patients who may have issues accessing medications. A better understanding of dermatologists that targeted TYK2 inhibition is not the same as pan-JAK inhibition so there is no reluctance to use this class of effective therapies is key for the continued success.
Miller: When looking at the success of any drug, the importance of long-term safety and durability of response cannot be overstated. As a medical professional, long-term safety data helps me provide informed decision-making, minimizing risks and adverse effects that may occur with long-term use. The durability of a therapy fosters patient trust and adherence, leading to better disease management and improved quality of life. If deucravacitinib can provide this, it will hold a key place in the treatment paradigm for patients with psoriasis. Reimbursement or access to the drug will always be critical, because a drug can be the safest and most efficacious on the market, but if my patients cannot get it, the company has failed.
Reference
1. US Food and Drug Administration approves Sotyktu (deucravacitinib), oral treatment for adults with moderate-severe plaque psoriasis. News release. Bristol Myers Squibb. September 9, 2022. Accessed August 14, 2023. https://news.bms.com/news/details/2022/U.S.-Food-and-Drug-Administration-Approves-Sotyktu-deucravacitinib-Oral-Treatment-for-Adults-with-Moderate-to-Severe-Plaque-Psoriasis/default.aspx






