- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Dapsone gel may prove viable in truncal acne
Author(s):
Pilot study suggests topical gel effective as monotherapy for acne vulgaris of the trunk.
Pilot study suggests topical gel effective as monotherapy for acne vulgaris of the trunk. (©OksanaVolina/Shutterstock.com)
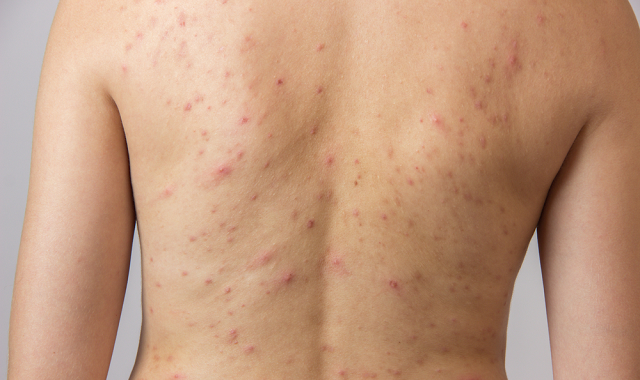
Management of acne vulgaris affecting the chest and/or back has received limited attention in clinical studies as most research have focused on managing acne vulgaris of the face.
However, at least half of all individuals affected by facial acne vulgaris also have acne vulgaris of the trunk and many want their trunk treated as well as their face, says researcher James Del Rosso, M.D., adjunct clinical professor at Touro University Nevada in Henderson, Nevada.
Oral therapy is often perceived to be a necessary part of the treatment regimen due to the extensive area involved.
In cases of severe inflammatory and nodular acne vulgaris, this is a reasonable approach, said Dr. Del Rosso, who also works for JDR Dermatology Research and Thomas Dermatology in Las Vegas, Nevada, but for other patients, except in cases warranting oral isotretinoin, it is recommended that the oral agents are combined with a topical regimen, particularly when the oral treatment is an antibiotic.
However, he points out: “Many cases of truncal acne vulgaris can be mild to moderate in severity and thus could potentially respond adequately to a topical regimen as initial treatment or as maintenance therapy after discontinuation of oral antibiotic therapy.”
Del Rosso’s research group conducted a 16-week, open-label, pilot study to test the efficacy and safety of dapsone 7.5% topical gel (Aczone gel 7.5%, Allergan Pharmaceuticals, Irvine, California, USA) as monotherapy in the management of moderate truncal acne vulgaris (truncal acne severity score of at least 3) involving the chest and back in 20 patients.
The results published in the Journal of Clinical Aesthetic Dermatology show that dapsone 7.5% topical gel was well-tolerated and well-accepted by study patients and associated with significant improvements in acne severity based on the Investigator Global Assessment scale and lesion counts.
The patients in the study were asked gently wash and pat dry affected areas of skin and apply a pea-sized amount of dapsone 7.5% gel in a thin layer once daily day for 16 weeks. They were seen in the clinic at baseline, and weeks four, 10, and 16.
At the start of the study acne vulgaris on the trunk was rated as moderate in 16 patients (80%) and severe in four (20%). At the end of 16 weeks, 55% of these 20 patients had achieved a two-grade improvement in severity and 45% were rated as clear or almost clear.
There were progressive declines in inflammatory lesion counts, non-inflammatory (comedonal) lesion counts, and total lesion counts over the course of the study, and these decreases were statistically significant at all time points. By week 16, inflammatory lesions, non-inflammatory (comedonal) lesions, and total lesions had decreased by 74% (p=0.0001), 69% (p=0.0002), and 72% (p=0.0001) respectively.
Four adverse reactions were reported in three subjects. Three were deemed unrelated to the treatment under investigation (leg abrasions with infection, oral surgery, sty), and one to be related (sunburn).
There were 15 reports of erythema, dryness, oiliness, pruritus, or burning; ten of these were rated mild or trace and had resolved to absent by the end of study, one was rated moderate and also resolved by the end of study, three rated as trace were unresolved at the end of the study, and one patient with a reaction rated as trace was lost to follow-up. Over the 16 weeks, five patients were lost to follow-up but their data were still included in the effectiveness and safety analyses.
A patient questionnaire was completed by the 15 patients who completed the study which revealed that the preferred topical therapy was a gel, followed by lotions and creams. Ointments and sprays were the least preferred. Most patients said preferred the dapsone 7.5% topical gel tested in the study over previous topical medications they had tried, with most rating it as good or excellent in terms of easy to apply over a large area, absence of smell, and lack of stickiness/greasiness.
“The results of this pilot study suggest that dapsone 7.5% gel is a viable option to add to the armamentarium for treatment of truncal acne vulgaris. A large-scale randomized controlled trial evaluating this agent for truncal acne vulgaris would be helpful in further defining its efficacy in this population of patients with vulgaris,” Dr. James Del Rosso said.
REFERENCES
Del Rosso JQ DO, Kircik L, Tanghetti E. Management of Truncal Acne Vulgaris with Topical Dapsone 7.5% Gel. J Clin Aesthet Dermatol. 2018 Aug; 11(8): 45–50.