- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Cysteamine Isobionic-Amide Complex Demonstrates On Par Efficacy, Onset of Action in Melasma as Kligman's Formula
Author(s):
Researchers compared the potent depigmenting agent with modified Kligman's formula, considered the gold standard therapy for melasma.
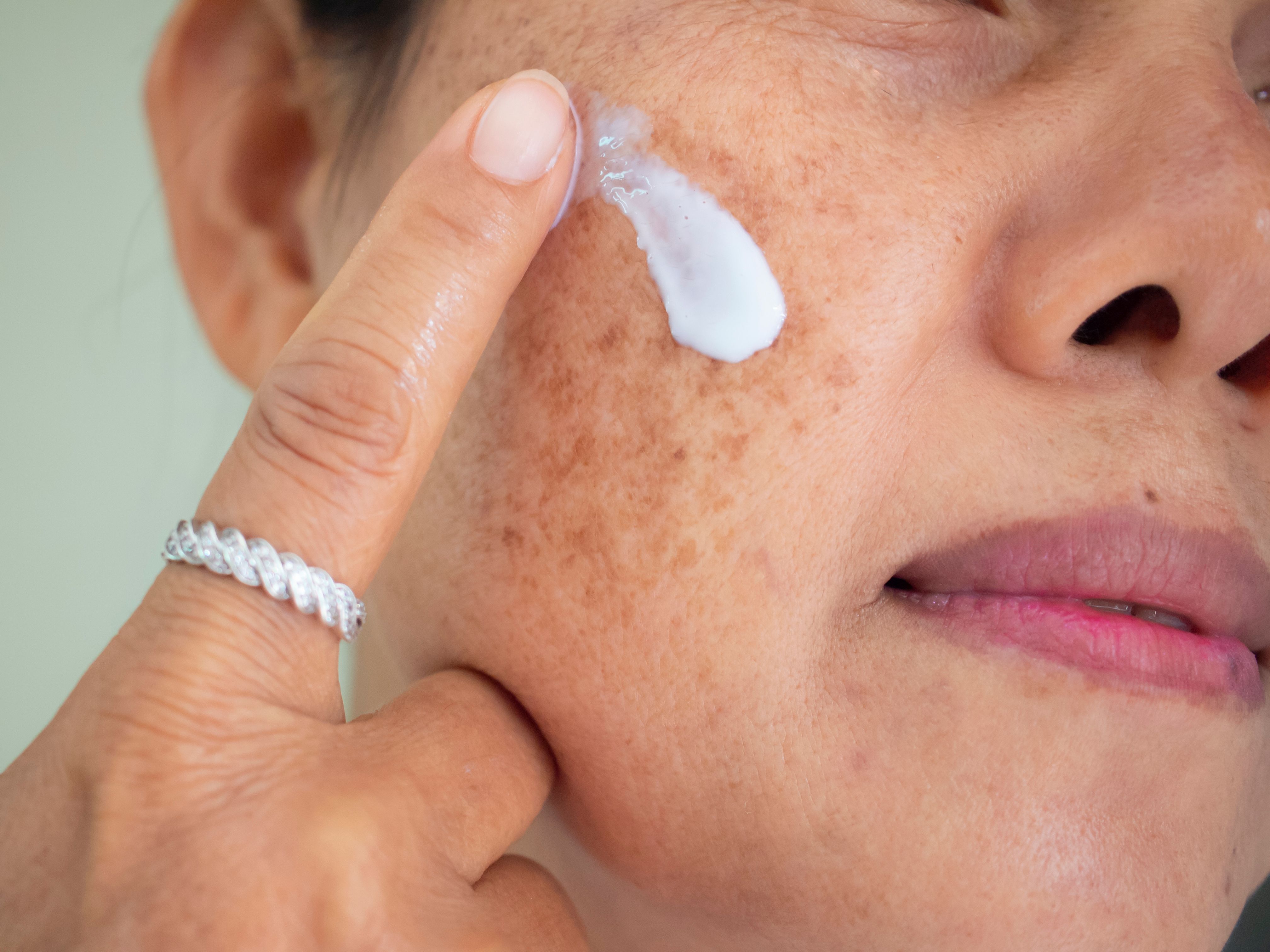
Cysteamine isobionic-amid (Cyspera; Scientis) complex, a depigmenting agent, exhibited on par efficacy and onset of action in melasma as with the gold standard therapy, Kligman's formula, in a recent study.1
The double-blind, randomized, and placebo-controlled trial, published in the Journal of Drugs in Dermatology (JDD), sought to compare the onset of action and efficacy of the complex with Kligman's formula. Study authors Sachdev et al noted that despite its role as the standard treatment for this pigmentary disorder, the prolonged use of modified Kligman's formula is not recommended in this indication due to adverse effects.
In total, 80 patients with melasma were included in the study. Upon inclusion, patients were randomized into 1 of 3 treatment groups, including treatment with cysteamine-isobionic amide, a placebo, or modified Kligman's formula.
At baseline and weeks 4, 8, and 16, researchers conducted spectrophotometric evaluations and examined patients using the modified Melasma Area Severity Index (mMASI).
Patient-reported metrics, including patient feedback, quality of life scores, and levels of satisfaction, were also collected and taken into account.
Both treatments showed significant pigment correction and evening of skin tone, with no notable difference reported between them. The cysteamine isobionic-amide complex exhibited rapid onset of action comparable to the modified Kligman's formula, significantly reducing melasma severity and improving skin condition by week 16.
Patients treated with the cysteamine isobionic-amide complex reported higher satisfaction levels and improved quality of life, suggesting it as a promising long-term alternative to modified Kligman's formula, potentially offering superior patient experience and safety.
However, the cysteamine isobionic-amide group experienced significantly greater improvements in quality of life at week 8, which continued to improve by week 16 compared to the modified Kligman's formula group. Patients also reported higher satisfaction with the cysteamine isobionic-amide treatment.
"As Scientis pigmentation authority behind Cyspera, I'm proud to share findings from our recent publication in the JDD February 2024 edition, Volume 23, Issue 2 which meticulously compares our new Cysteamine Isobionic-Amide complex with the gold standard in skin lightening," said Behrooz Kasraee, MD, chief scientific officer of Scientis, in a news release.2 "Results show Cyspera's effectiveness, with 96% of patients experiencing significant pigmentation reduction and improved quality of life, reinforcing our commitment to safe, effective hyperpigmentation solutions."
Study authors expressed excitement and promise at the data's publication.
"Our study marks a pivotal moment in the management of hyperpigmentation, introducing a promising alternative that aligns with the needs and preferences of patients," they said.2 "This publication underscores our dedication to pushing the boundaries of what's possible in hyperpigmentation answer, striving to improve the lives of those affected by pigmentation disorders. We extend our sincerest gratitude to our research team, study participants, and the broader scientific community for their invaluable contribution to this undertaking."
References
- Sachdev M, Grimes PE, Callender V, et al. Cysteamine isobionic-amide complex versus Kligman’s formula for the treatment of melasma: equal efficacy and rapid onset of action. January 24, 2024. Accessed February 23, 2024. doi:10.36849/JDD.7428
- Innovative hyperpigmentation solutions: Insights from our latest research. News release. PR Newswire. February 22, 2024. Accessed February 23, 2024. https://www.prnewswire.com/news-releases/innovative-hyperpigmentation-solutions-insights-from-our-latest-research-302067940.html