- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
Continued Efficacy of Dupilumab for Children with AD
Author(s):
Researchers not only confirmed the rapid and sustained efficacy of the drug, but also observed an increased onset of action for children under 6.
Image Credit: © Марина Терехова - stock.adobe.com
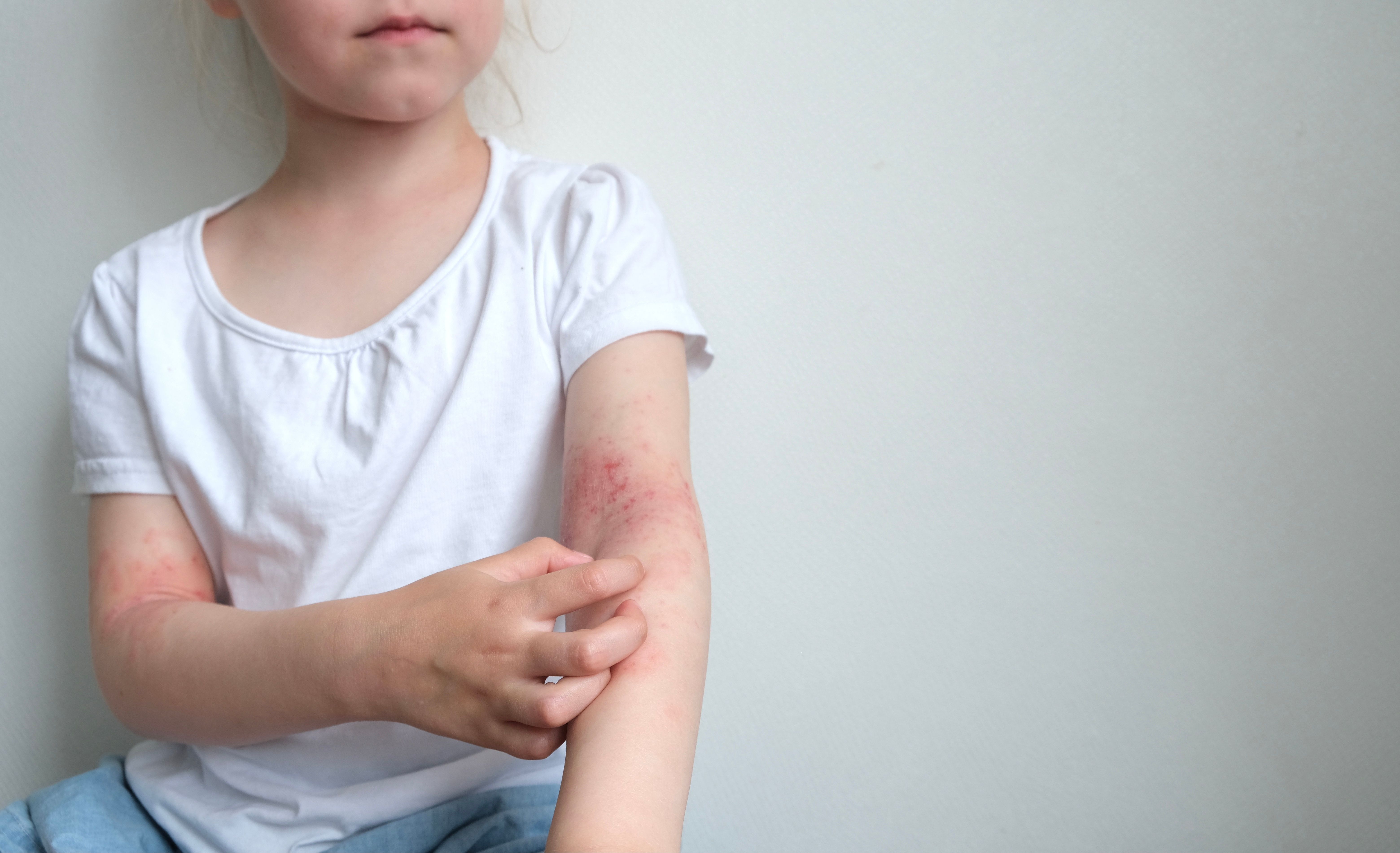
After a case series of 71 children and adolescents with atopic dermatitis (AD) treated with dupilumab (Dupixent; Sanofi)was recently published,1 a retrospective study out of DrBalmis University Hospital in Alicante, Spain, contributed evidence from 32 more young patients. The results of the secondary study provided further evidence for the “rapid and sustained efficacy of dupilumab” for children aged 4 to 14 with moderate to severe AD.2
Methods and Results
Throughout the study, researchers collected information on epidemiology, clinical and treatment variables, comorbidities, Eczema Area Severity Index (EASI), SCORing AD (SCORAD), Investigator Global Assessment (IGA), Body Surface Area (BSA), pruritus, sleepNumerical Rating Scale (NRS), quality of life, drug dose, and adverse events.
During follow-up, researchers found the mean initial EASI of 21.2 (Standard Deviation (SD) 10.6) had dropped to 8.5 (SD 6.7) at 4 weeks and 6.1 (SD 4.1) at 12 weeks. At week 4, they reported that 11 of the 32 children evaluated (34.4%) achieved EASI 75, a score that increased to 52.2% in the 23 children evaluated at week 12.
Researchers reported that over longer-term follow-up, improvement was sustained, with mean EASI being 3.0 (SD 3.1) at week 24, 5.8 (SD 8.1) at week 52, and 1.9 (SD 2.07) at week 100. They also observed improvement in the SCORAD index, in pruritus and sleep NRS, and in atopic comorbidities. The study reported mild adverse events, the most frequent being pain at the injection site (n = 13; 40.6%) which led to treatment being suspended in 2 cases. Researchers stated that one child stopped treatment reporting a lack of efficacy, and another for unknown reasons. The study stated that 8 children required treatment optimization (increasing the dosing interval) because of good response, and 4 required a higher dose.
In general, researchers wrote that improvement was rapid, with 34.4% of the children achieving EASI 75 at week 4% and 52.2% at week 12. When comparing the improvement between the week of initiation and the following weeks, the study reported that differences in the reduction of EASI were already significant at week 4 and were maintained over the following weeks. They reported a borderline significant reduction in EASI from week 4 to week 12 and to week 24. Researchers found no significant reduction from week 24 to weeks 52 and 100.
The study found that 62.5% of children under 6 years of age reached PASI 75 at week 4, suggesting that the onset of action for the drug may be even faster in that age group. This is a slightly higher percentage than previously reported.3Researchers reported that SCORAD, pruritus, and sleep scores dropped in parallel with EASI, also noting a clinical improvement in asthma and rhinitis, leading patients to no longer require the drugs to control those symptoms. As for adverse events, only a few cases of conjunctivitis or facial erythema and pain at the injection site were observed.
Conclusion
This study provides further evidence for the “rapid and sustained efficacy of dupilumab” in a series of children aged 4 to 14 years with moderate to severe AD. With the exception of pain at the injection site, the researchers found no major safety concerns. Investigators identified that the main limitations of this study were the small sample size, its single-center nature, and the differing follow-up across the series, as children started the drug at different times and some values were missing from clinical records.
References
- Hosseini-Ashrafi M, Clayton TH, Herring M, et al. Real-world outcomes of children treated with dupilumab for moderate-to-severe atopic dermatitis: a single-centre retrospective observational UK study. Clin Exp Dermatol. 2024;49(6):578-583. doi:10.1093/ced/llae013
- Betlloch-Mas I, Armengol-García M, Jara-Rico N, et al. Atopic dermatitis in 32 children treated with dupilumab in real life. A new contribution. Skin Health Dis. 2024;e413. https://doi.org/10.1002/ski2.413
- Berna-Rico E, Fiz-Benito E, Busto-Leis JM, et al. Effectiveness and Safety of Dupilumab in Children Under 6 Years of Age with Moderate-to-Severe Atopic Dermatitis: A Retrospective Real-World Study. Dermatology. 2024;240(2):337-342. doi:10.1159/000535282






