- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Calcipotriene betamethasone foam phase 3 trial succeeds
Author(s):
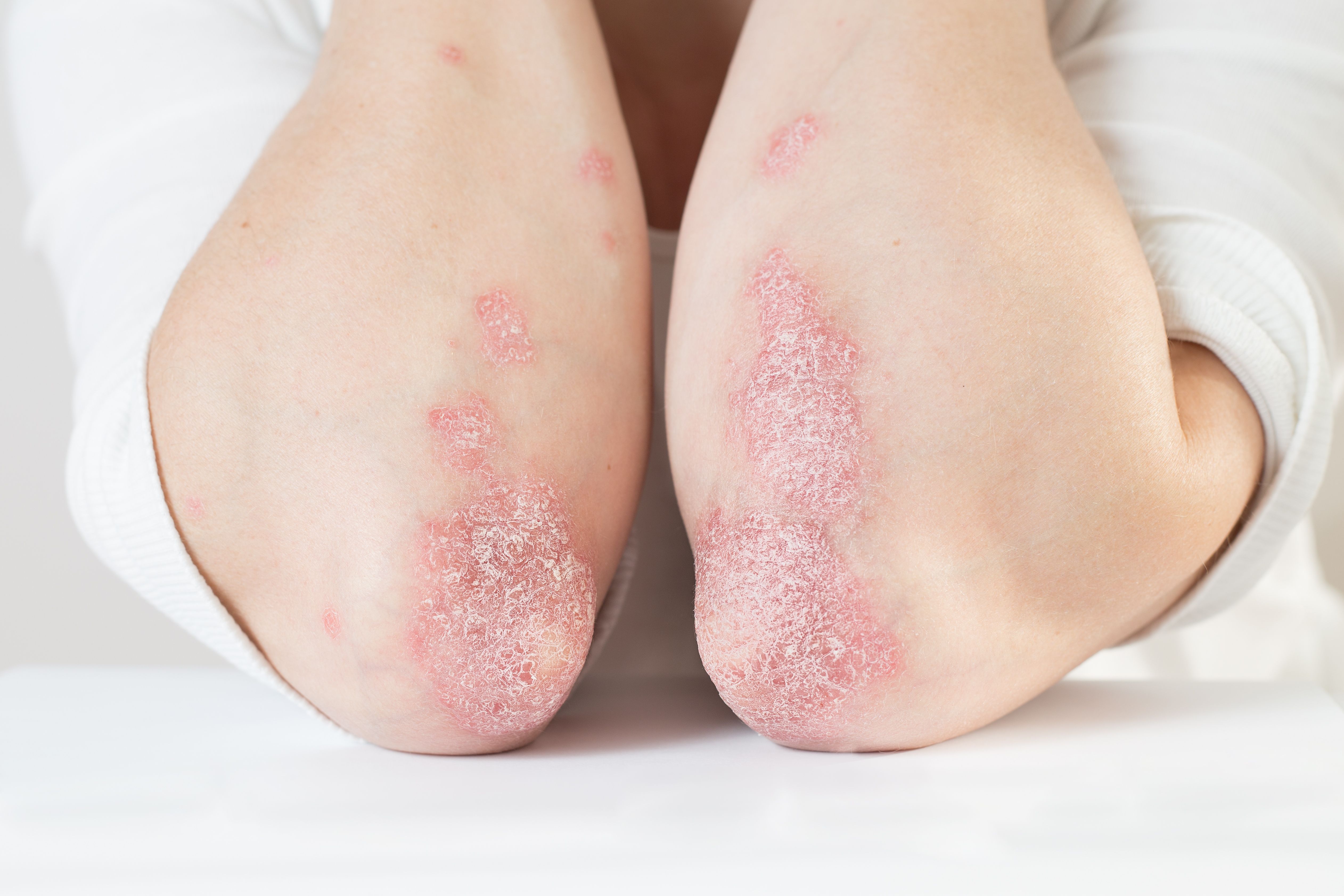
Twice-weekly treatment with calcipotriene-betamethasone diproprionate provides safe and effective maintenance treatment for plaque psoriasis, according to phase 3 data.
Twice-weekly Enstilar Foam (calcipotriene-betamethasone diproprionate, LEO Pharma) provides safe and effective maintenance treatment for plaque psoriasis, according to results of the PSO-LONG phase 3 trial.
To address psoriasis, says Mark Lebwohl, M.D., strong topical steroids are approved by the U.S. Food and Drug Administration (FDA — some for limited periods such as two to four weeks.
"Then the question becomes, what do you do for a disease that is long-term? How do you avoid steroid side effects such as stretch marks, telangectasia, cutaneous atrophy and adrenal suppression? We know that long-term steroid use will create those side effects."
He is Waldman Professor and Chairman, Kimberly and Eric J. Waldman Department of Dermatology, Icahn School of Medicine at Mount Sinai, and principal PSO-LONG investigator.
Combining calcipotriene with betamethasone diproprionate (a class II steroid) creates the efficacy of a superpotent steroid while avoiding the associated risks, he says. In PSO-LONG, investigators randomized patients who achieved investigator global assessment scores of zero or one (clear or almost clear) after four weeks' daily open-label use of calcipotriene-betamethasone foam to twice-weekly foam or vehicle for 52 weeks.
After the initial month of treatment, 521 of 650 patients achieved treatment success; 545 entered the double-blinded maintenance phase, which include monthly follow-up visits. Patients who experienced breakthrough flares during this period were put on daily active treatment until clearance before returning to twice-weekly calcipotriene-betamethasone foam or placebo.
Regarding median time to relapse, says Dr. Lebwohl, calcipotriene-betamethasone foam showed a clear advantage — 56 (95% confidence interval/CI 34-59) days versus 30 (95% CI 29-31) days for vehicle. The corresponding times until 75% of patients experienced a first relapse were 169 (95% CI 140-197) days and 57 (95% CI 55-61) days.
Over the course of a year, the investigational foam furthermore provided an average of 34.5 more remission days versus placebo (256.5 versus 222 days, respectively). The mean percentage of days in remission was 70% for calcipotriene-betamethasone foam versus 61% for placebo. The average number of relapses per year was three for active treatment versus 4.7 for placebo.
"Keep in mind that people in the vehicle group were allowed to use rescue treatment," he says.
This fact boosted remission numbers in the vehicle cohort. Additionally, active treatment reduced the relapse rate 46% (95% CI 37%-54%) versus vehicle.
"And there were more rebounds, meaning the disease came back worse, in the vehicle group," says Dr. Lebwohl.
As a drawback of the study's design, he says that with a year-long topical medication trial, PSO-LONG investigators expected a fairly significant number of dropouts — 251 patients (46.1%) completed the study.
"In terms of safety, there were no steroid-related side effects with this prolonged regimen. The number of adverse events was similar between the two arms," Dr. Lebwohl says.
In the actively treated cohort, one patient each experienced chorioretinopathy and skin pain, which investigators adjudicated to be possibly related and unrelated to treatment, respectively. No patients experienced calcium-related side effects.
"Across the board," he says, "the results are excellent."
Until now, says Dr. Lebwohl, guidelines in package inserts of topical steroids indicated for psoriasis allowed medication use for a limited time.
"Once that period of time is over, what do you do with a patient with a chronic disease? Can you keep treating them? Use the medication twice a week or go to a weekend-only regimen?" he asks.
No one really knew what to do, he says.
"This is the first time we have a large, multicenter, double-blinded study in which we proved that using twice-weekly calcipotriene-betamethasone diproprionate foam for a year does not result in more side effects but does result in better outcomes. It gives everyone a sensible, practical regimen to follow," Dr. Lebwohl says.
DISCLOSURES
Dr. Lebwohl is a consultant and investigator for LEO, and he works with most other companies that market psoriasis treatments. All payments for research go to his employer, the Icahn School of Medicine at Mount Sinai, and he receives no personal direct compensation from the manufacturers of psoriasis therapies.
REFERENCE
LebwohlM, Lacour JP, Liljedahl M, et al. Long-term proactive management of psoriasis vulgaris with fixed-dose combination of calcipotriene 0.005% and betamethasone dipropionate 0.064% foam; results of a phase III randomized controlled trial. Poster 18223. American Academy of Dermatology VMX. June 12, 2020.