- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Brodalumab Demonstrates Quality of Life, Mental Health Improvement in Patients Regardless of Prior Biologic Use
Author(s):
A poster presented at the 2023 SDPA Annual Summer Dermatology Conference revealed statistically significant improvements among patients with psoriasis receiving brodalumab treatment.
Patients with psoriasis who were treated with brodalumab experienced statistically significant improvements to their overall quality of life (QOL) and mental health, regardless of the patients’ prior history of biologic use, according to a poster1 presented at the 2023 Society of Dermatology Physician Assistants (SDPA) Annual Summer Dermatology Conference in Boston, Massachusetts.
quayside/AdobeStock
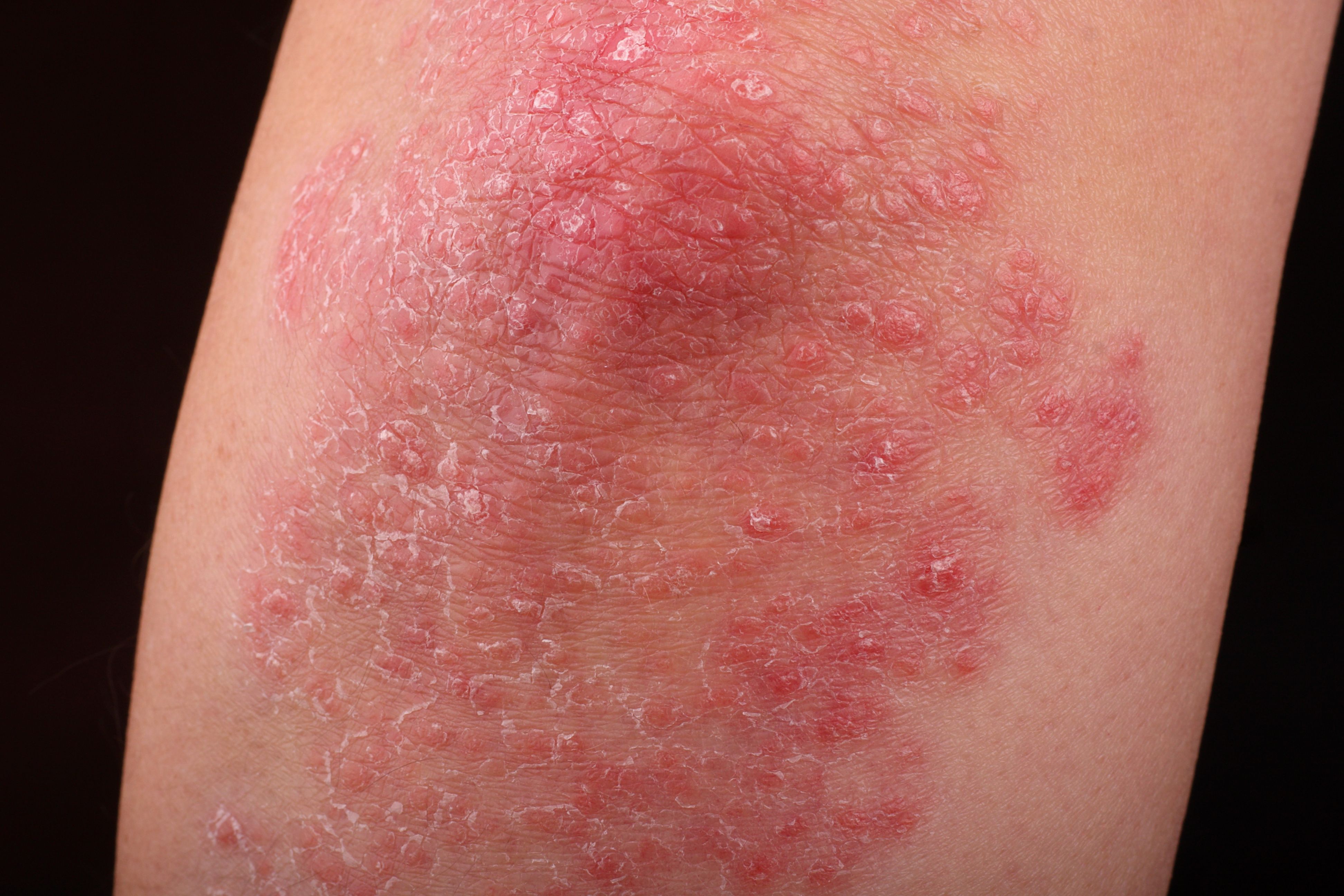
In the post hoc analysis, researchers sought to evaluate the patient-reported QOL and mental health effects from the AMAGINE-1 trial, wherein researchers had assessed efficacy, safety, withdrawal, and retreatment of patients with moderate-to-severe plaque psoriasis and brodalumab treatment. They cited the association of psoriasis with high rates of depression, anxiety, and other mental health concerns.
AMAGINE-1 included 305 patients with prior biologic exposure and 356 patients with prior biologic exposure. Of these patients, some were randomized to receive a 210 mg dose of brodalumab, while others received a 140 mg dose or a placebo for a 12-week period. Patients receiving the placebo treatment were then assigned to receive 210 mg of brodalumab, while patients who had already been receiving brodalumab were rerandomized.
Researchers measured patient QOL using the Dermatology Life Quality Index (DLQI) while also assessing mental health conditions, such as anxiety and depression, by using the hospital anxity and depression scale (HADS).
At baseline of the trial, patients with prior exposure to biologics had an average DLQI score of 14.2, while patients without prior exposure had an average score of 14.1.
Compared to patients receiving the placebo, patients receiving brodalumab experienced statistically significant improvements to average DLQI. Patients receiving brodalumab experienced a decrease in average DLQI score down to 2.3, while patients receiving the placebo had an increase, bringing their average DLQI score up to 15.2. This improvement was also evident among patients without prior biologic exposure.
“Regardless of prior biologic exposure, there was numeric reduction in means HADS anxiety scores with brodalumab from baseline,” according to Armstrong et al. “In the placebo group, mean HADS anxiety scores were unchanged from baseline to week 12.”
This trend was also evident in the average HADS depression scores of patients.
“Patients with psoriasis can experience a profound psychsocial burden that may negatively influence QOL,” according to Armstrong et al. “Brodalumab demonstrated improvements in QOL and symptoms of mental health in patients with psoriasis regardless of prior biologic exposure.”
Reference
- Armstrong A, Glick B, Bhutani T, Jacobson A. Quality of life and mental health of patients stratified by prior biologic exposure: post hoc analysis of brodalumab. Poster presented at the 2023 Society of Dermatology Physician Assistants (SPDA) Annual Summer Dermatology Conference; June 22-25, 2023; Boston, MA.






