- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
BRAVE Trials: 52-Week Baricitinib Data Demonstrate Continued Improvements in Alopecia Areata
Author(s):
The proportion of patients who achieved a SALT score < 10 jumped from 24.9% at 36 weeks to 28.9% at 52 weeks in the BARI 4-mg arm and 11.8% to 15.3% in the BARI 2-mg arm.
(Adobe Stock/Alex Papp)
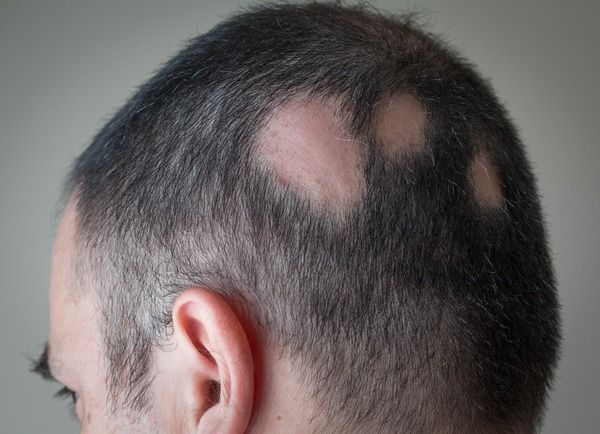
The US Food and Drug Administration approved baricitinib as the first systemic treatment for patients with alopecia areata (AA) in June 2022 based off 36-week data from 2 phase 3 randomized, double-blind, placebo-controlled trials—BRAVE-AA1 and BRAVE-AA2.
In a poster1 presented at Winter Clinical Miami, held February 17-20, 2023, in Miami, Florida, investigators shared integrated 52-week long-term safety and efficacy data from BRAVE-AA1 and BRAVE-AA2 (NCT03570749 and NCT03899259, respectively), in which baricitinib (Olumiant; Eli Lilly) demonstrated continued efficacy that resulted in an increased proportion of patients achieving eyelash, scalp, and eyebrow hair regrowth.
The trials included individuals > 18 years old (< 60 years for males and < 70 years for females) who experienced hair loss that involved > 50% of the scalp and whose current AA episode ranged from > 6 months to < 8 years. To be eligible for study enrollment, individuals must not have experienced any spontaneous improvement in the 6 months prior to screening.
Participants were randomized to either placebo (PBO; n = 345), baricitinib (BARI) 2-mg once a day (QD) (n = 340), or BARI 4-mg QD (n = 515). Participants in the BARI treatment arms continued treatment through 52 weeks, while non-responders in the PBO group were rescued at 36 weeks.
The primary outcome at 36 weeks was a Severity of Alopecia Tool (SALT) score < 20, with continued observation through week 52. The SALT assessment is meant to be objective and divides the scalp into quadrants in order to analyze hair loss. The score factors the weighted sum of the percentage of hair loss in each of those 4 quadrants and ranges from 0 (no hair loss) to 100 (complete hair loss).
At 52 weeks, investigators also assessed the proportion of patients who achieved a SALT score ≤ 10, as well as the proportion of patients who achieved Clinician-Reported Outcome (ClinRO) Measure for Eyebrow Hair Loss and Eyelash Hair Loss scores of 0 or 1 with ≥ 2-point improvement from baseline among patients with baseline scores ≥ 2. ClinRO scores of 2 or 3 indicate significant hair loss in eyebrows or eyelashes, with a score of 3 meaning no notable eyebrows or eyelashes present.
Looking at the pooled population of both BRAVE-AA trials, baseline characteristics were comparable across the PBO group and both treatment arms in terms of mean age (37.2 [12.6] PBO; 38.4 [12.9] BARI 2-mg; 37.1 [13.0] BARI 4-mg) and female gender (60.0% [n = 207]; 62.4% [n = 212]; 60.0% [n = 309], respectively). Participants were majority white and Asian across all 3 arms, with an average duration of AA onset of 12.26 years.
Mean SALT scores at baseline were 84.8 (17.8) in the PBO group, 86.3 (18.0) in the BARI 2-mg group, and 85.1 (18.1) in the BARI 4-mg group. The percentage of patients with ClinRO Eyebrow Hair Loss scores of 2 or 3 in each of the 3 groups were 69.4%, 70.6%, and 68.3%, respectively. The percentage of those with ClinRO Eyelash Hair Loss scores of 2 or 3 were 54.7%, 58.8%, and 60.1%, respectively in the PBO, BARI 2-mg, and BARI 4-mg arms.
Investigators previously reported that more than 30% of patients in the BARI 4-mg arm achieved a SALT score < 20 after 36 weeks of treatment, while more than 31% in that arm also experienced > 2-point improvements from baseline in ClinRO Eyebrow Hair Loss and Eyelash Hair Loss scores (0 or 1).
After 52 weeks of treatment with either 2- or 4-mg of the oral Janus kinase (JAK) 1 and 2 inhibitor, the proportion of patients who achieved SALT Score ≤ 20 and a SALT score ≤ 10 increased, although patients in the 4-mg arm reported better outcomes.
At 52 weeks, 39.0% of patients in the BARI 4-mg group achieved a SALT score of < 20 compared with 34.0% after 36 weeks. Patients in the 2-mg arm still reported an increase, from 19.7% at 36 weeks to 22.6% at 52 weeks.
The proportion of patients who achieved a SALT score < 10 jumped from 24.9% at 36 weeks to 28.9% at 52 weeks in the BARI 4-mg arm and 11.8% to 15.3% in the BARI 2-mg arm.
After the long-term extension, patients also experienced improvements in both ClinRO Eyebrow and Eyelash Hair Loss scores. The proportion of patients who scored a 0 or 1 in Eyebrow Hair Loss at 52 weeks with a > 2-point improvement from baseline and who scored > 2 at baseline increased from 33.0% at 36 weeks to 44.1% at 52 weeks in the BARI 4-mg arm, and 15.8% to 22.9% in the BARI 2-mg arm.
For Eyelash Hair Loss, the proportion of patients who scored 0 or 1 jumped from 33.6% at 36 weeks to 45.3% at 52 weeks in the BARI 4-mg group and 12.0% to 25.5% in the BARI 2-mg group.
Investigators reported safety data up to 128 weeks for BRAVE-AA1 and 108 weeks in BRAVE-AA2, with findings consistent with the known safety profile of BARI.
Reference:
Kwon O, Senna MM, Sinclair R, et al. Long-term efficacy and safety of baricitinib in patients with severe alopecia areata: week 52 results from BRAVE-AA1 and BRAVE-AA2. Poster presented at Winter Clinical Miami 2023 meeting; February 17-20, 2023; Miami Beach, FL; Accessed February 15, 2023.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














