- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Botulinum Toxin Type A Unparallel Improvements to Skin Quality
Author(s):
Researchers investigated the injection’s impact on the upper face.
Botulinum toxin type A (BoNT-A) injections are capable of achieving unparallel improvements to overall skin texture and dynamic wrinkles in the upper face, according to new research.
Valua Vitaly/AdobeStock
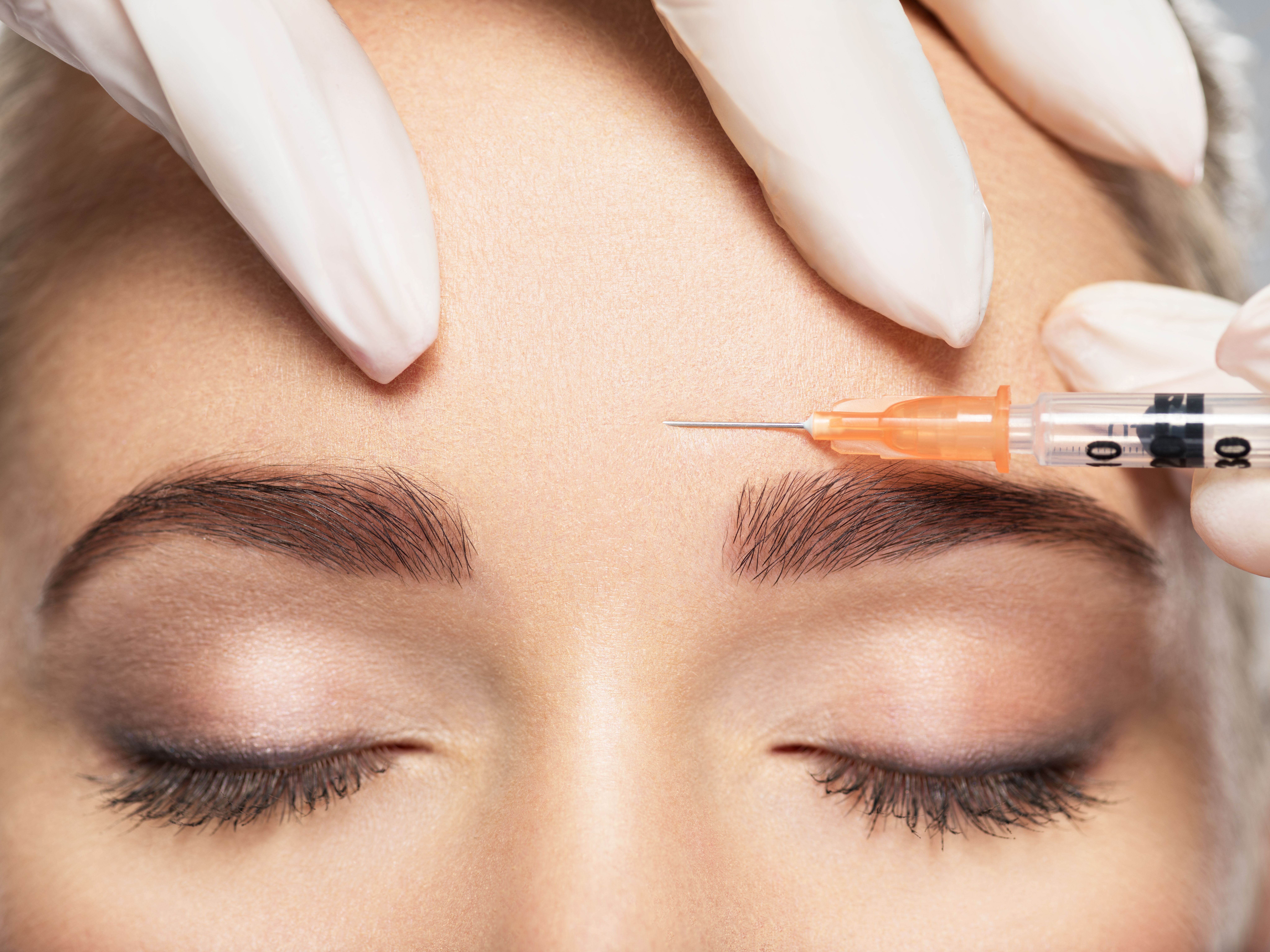
In a recent study,1 researchers sought to assess improvements in these indications, citing a lack of quantitative and comprehensive analysis of skin changes post BoNT-A treatment. They stated that previous research and evidence may suggest long-term, progressive improvements to patient skin quality, including the ability to regulate skin remodeling and increase the production of sebum.
Study participants (n=24) were 30.5 years of age on average and ranged in age from 18 to 65 years old. All participants had moderate or severe dynamic wrinkles (forehead, glabellar, or lateral periorbital) and provided written consent to participate.
Prospective participants were excluded from consideration if they had received filler or neuromodulator injections within 1 year prior to the start of the study, had visible scarring or infections in the upper face, hypersensitivity to BoNT-A, or were pregnant or lactating.
Participants received Botox (Allergan) injections. Prior to injection, an experienced plastic surgeon applied compound lidocaine treatment 30 minutes in advance.
For injections given in the forehead region, participants received 0.5 units subcutaneously on each site, as indicated in a 2-row diagram formulated by researchers. In cases of glabellar wrinkles, participants received 4 units per site, with an additional 2 units injected into the center of the glabellar region. 2 units were injected into each lateral periorbital site impacted by wrinkles.
Researchers collected data related to dynamic wrinkle severity (DWS), fine wrinkle severity (FWS), pore volume (PV), and skin texture roughness (STR) at baseline and additional follow-up visits. These visits took place at 1 week, 1 month, 3 months, and 6 months post treatment.
They performed the Shapiro-Wilk test to analyze the collected data for normality. Additionally, they also completed a Wilcoxon matched-pairs signed rank test for detecting differences between baseline and respective follow-up visits, and formulated an improvement rate index to account for improvement over time.
At the study’s conclusion, researchers observed significant DWS improvement in all 3 regions as early as 1 week post-injection. This difference decreased gradually over time, falling below significance at the 3-month mark, but was still noted at 6 months.
FWS improvement in all 3 regions was significant at 1 month post-injection, and was maintained through month 6.
PV was significant only in the frontal area at the 1 month mark. The glabellar and lateral periorbital sites experienced significant improvement at 1 week. Pore shrinking persisted for 5 months post-injection.
The glabellar and frontal areas experienced significant STR improvement in as early as 1 month and was sustained for 5 months. In the lateral periorbital region, improvement was noted at the 1 week mark. In the periorbital injection area, this improvement persisted for 6 months.
While no severe adverse effects were reported, 3 participants experienced mild injection-site bruising. This resolved in as little as 2 weeks.
Potential limitations included a reduced sample size and shorter study period. Additionally, researchers did not perform a subpopulation analysis or conduct basic experiment verification.
“With objective assessment, the reduction of dynamic wrinkles as well as the improvement of skin quality was observed after BoNT-A treatment. Unparallel improvement patterns of dynamic wrinkles and skin quality were presented,” study authors wrote. “The former presents a slower onset but longer duration while the latter exhibits a more rapid onset but relatively shorter duration. The findings of this study provided new insights for facial BoNT-A treatment.”
Reference
- Sun Y, Li Y, Zhang Y, et al. Unparallel improvement patterns of dynamic wrinkles and skin quality after botulinum toxin type a treatment on the upper face. Skin Res Technol. 2023;29(3). doi:10.1111/srt.13309





