- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Biophotonic system earns CE mark
Author(s):
Kleresca's new biophotonic technology received CE mark of approval, which will allow it to expand in the U.S., Canada, Australia and throughout Europe.
Kleresca’s biophotonic system includes the multi-LED Kleresca lamp. It uses pre-programmed wavelength settings and a photoconverter gel to convert light waves from the lamp into dynamic, pulsing fluorescent energy to stimulate the skin’s repair mechanism. Kleresca is expanding the biophotonic technology into the US., Canada, Australia and Europe. (Photo courtesy of Kleresca)
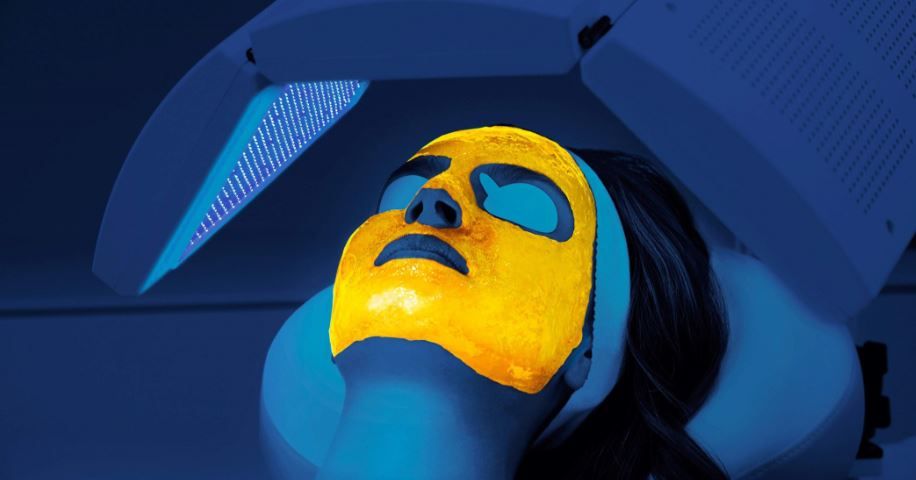
Kleresca, a U.K.-based company offering biophotonic technology for various skincare treatments, recently received CE mark approval, which will allow it to expand into the U.S., Canada, Australia and throughout Europe, according to a news release.
The company’s technology uses a propriety multi-LED lamp designed with preprogrammed wavelength settings in combination with a “specially formulated photoconverter gel.” Chromophores in the gel then convert the light waves into “pulsing fluorescent energy,” resulting in stimulation of the skin’s repair mechanisms, according to the company.
Kleresca’s system is used for addressing acne, rosacea and skin rejuvenation. The company operated under the CE mark of one the brands that founded it, LEO Pharma, until June.
Kleresca also offers a pre-post biophotonic treatment that prepares the skin for invasive or high-energy laser procedures. It claims to help increase collagen buildup, activate the skin’s regenerative processes, and reduce inflammation and erythema.






