- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
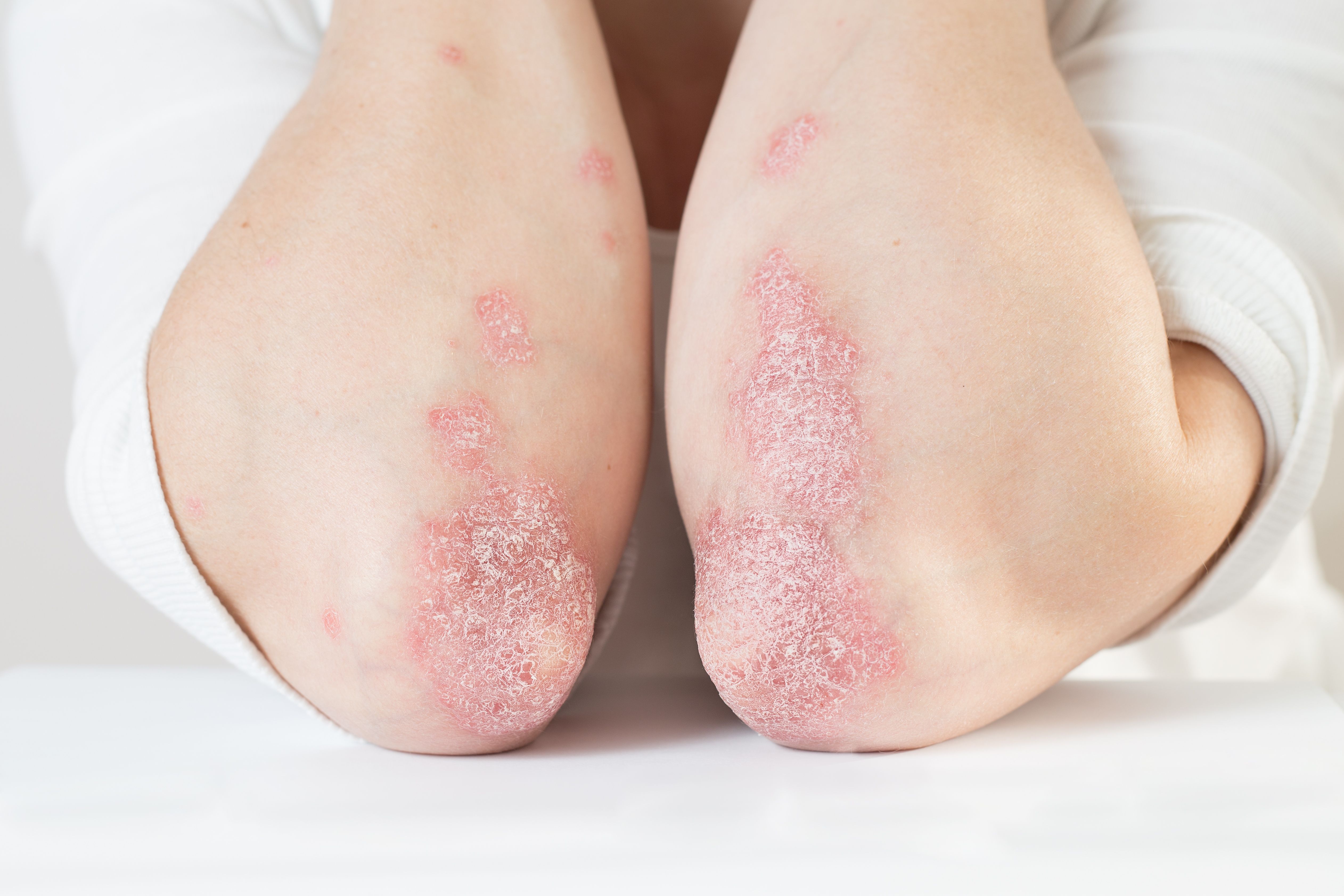
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Publication
Article
Dermatology Times
Biologics enter post-PASI era
Author(s):
With newer biologics consistently posting PASI improvements greater than 90%, dermatologists need to consider other factors when selecting a treatment. However, nuanced differences between the same drug class could be teased out, one expert says.
With newer biologic drugs for psoriasis generally offering excellent and near-equivalent efficacy, an expert says, choosing the best drug for a particular patient requires considering additional factors.
“In the old days,” says George Han, M.D., Ph.D., “you could say ustekinumab (Stelara, Janssen) works better than etanercept (Enbrel, Amgen), and ixekizumab (Taltz, Lilly) works better than ustekinumab. There were bigger differences stratifying the medications, so you might make your choice based on efficacy as the main factor. But nowadays that isn’t so true anymore.” He is system medical director and chief of teledermatology for Mount Sinai Health System and assistant professor of dermatology at the Icahn School of Medicine at Mount Sinai.
With newer biologics consistently posting Psoriasis Area and Severity Index (PASI) improvements greater than 90% in pivotal trials, says Dr. Han, dermatologists need to consider additional factors such as speed of onset, durability, safety, patient comorbidities and dosing regimens.
RELATED: Guidelines reflect continued popularity of oral treatments
Real-world comparative studies consistently show that ustekinumab response lasts longer than that of TNF inhibitors. Based partly on ustekinumab data, he adds, many dermatologists expect the interleukin (IL)-23 blockers guselkumab (Tremfya, Janssen), tildrakizumab (Ilumya, Sun Pharma) and risankizumab (Skyrizi, Abbvie) to perform well long-term, although these drugs do not yet have the long track record of ustekinumab.
“Among TNF alpha inhibitors, it appears to be adalimumab (Enbrel, Amgen), then infliximab (Remicade, Janssen), then etanercept as the least likely to have long-term continuation on the treatment,” he says.
In a comparison against ustekinumab and TNF inhibitors published in the February 2018 British Journal of Dermatology, secukinumab (Cosentyx, Novartis) fared worst.1
“It is important to note, however, that this is just one study cohort, and many factors impact real-world efficacy and durability of biologics,” Dr. Han adds.
Regarding long-term safety, says Dr. Han, TNF inhibitors are generally believed to be more immunosuppressive than newer medications.
“That’s especially relevant right now as everybody’s thinking about infectious risk,” he says. “Ustekinumab, IL-17 inhibitors and IL-23 inhibitors all look better than the TNF alpha inhibitors from a safety standpoint.”
It is well-known that IL-17 inhibition can worsen inflammatory bowel disease (IBD).
“It seems to be primarily IL-17A that drives the negative effects. Inhibiting IL-17A seems to knock out this regulatory function of IL-17 in the gut, which may worsen IBD,” he says.
With newer medications, though, even more nuanced differences between the same drug class could be teased out, says Dr. Han. A mouse study published in the June 2018 Nature Immunology showed that suppressing IL-17F could help IBD.2
“Consistently, blocking IL-17A in this animal model worsened IBD, while blocking IL-17F ameliorated it. Furthermore, when researchers blocked both IL-17A and F, it was helpful — it seemed that the function of IL-17F predominates over IL-17A,” Dr. Han says. “This is particularly relevant because a new dual inhibitor of IL-17A and F is coming to the market soon.”
Similarly, he says, blocking IL-23 is likely to help IBD as early phase trials of IL-23 inhibitors have shown promise.
Brodalumab (Siliq, Ortho Dermatologics) and ixekizumab provide the quickest response — PASI 50 in under two weeks. For patients with psoriatic arthritis (PsA), most dermatologists choose TNF inhibitors or IL-17 inhibitors. Guselkumab phase 3 data – the first released for an IL-23 inhibitor – are also fairly impressive in this regard, adds Dr. Han, who expects this drug to earn PsA approval soon.
REFERENCE:
1 Egeberg A, Ottosen MB, Gniadecki R, et al. Safety, efficacy and drug survival of biologics and biosimilars for moderate-to-severe plaque psoriasis. Br J Dermatol. 2018;178(2):509-519.
2 Tang C, Kakuta S, Shimizu K, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing T cells through modification of the intestinal microbiota. Nat Immunol. 2018;19(7):755-765.
DISCLOSURES:
Dr. Han has received research funds (grants paid to Mount Sinai) from and/or been a consultant for AbbVie, Athenex, Boehringer Ingelheim, Bond Avillion, Bristol-Myers Squibb, Celgene, Eli Lilly, Novartis, Janssen, LEO Pharma, MC2, Ortho Dermatologics, PellePharm, Pfizer, Regeneron, Sanofi Genzyme, SUN Pharmaceuticals and UCB.