- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Biologic Therapy Linked to Increased Density of Demodex Mites and Prevalence of Demodicosis in Patients With Psoriasis vs Controls
Author(s):
A cross-sectional study revealed an increase and heightened prevalence of Demodex and demodicosis in individuals with psoriasis receiving biologic therapy.
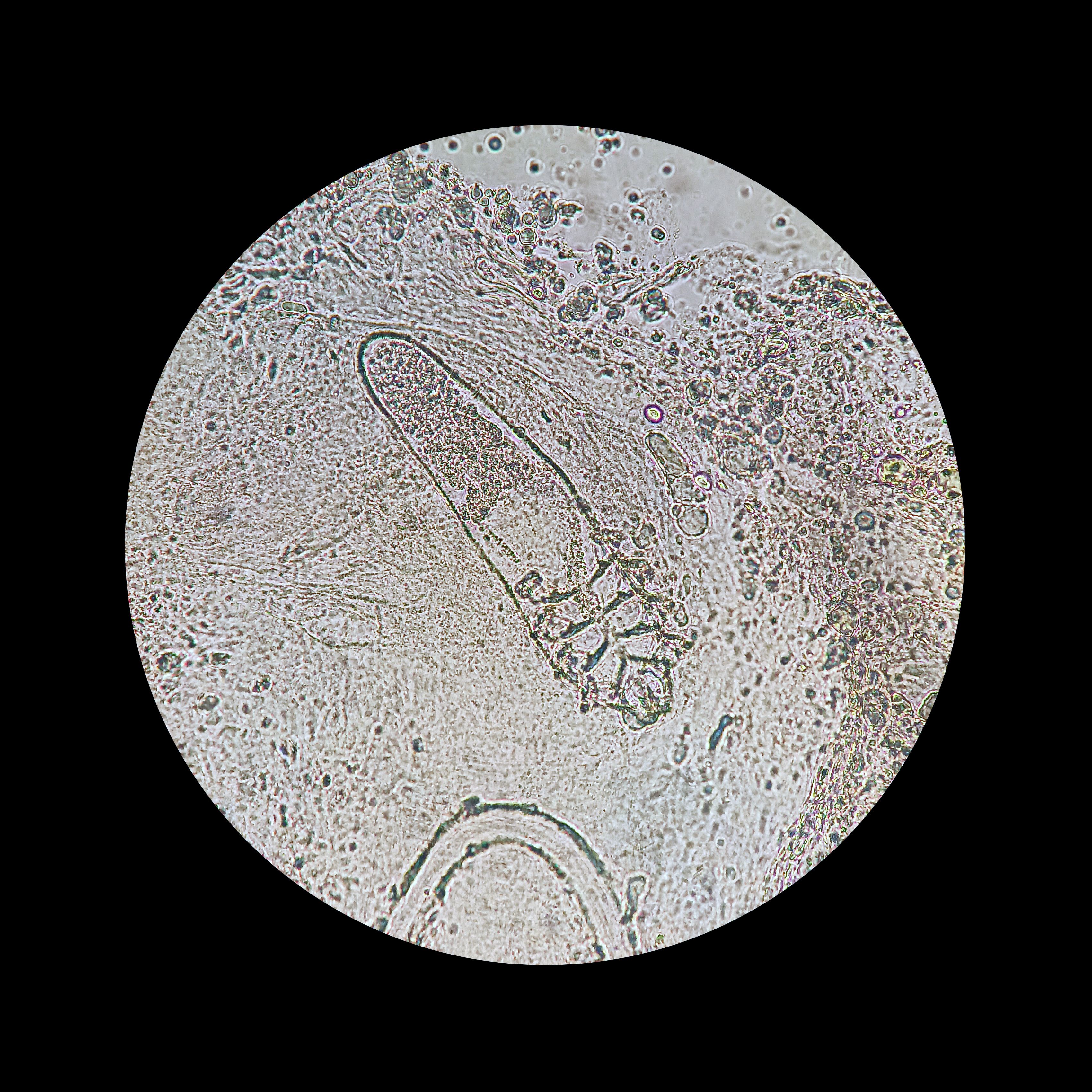
Demodex mites, initially described in 1842, are typically harmless inhabitants of human pilosebaceous units but can contribute to inflammatory skin conditions, termed demodicosis.1
Prior scientific literature has been indicative of an increase in Demodex density among patients with various immunosuppressive conditions. For example, research has pointed to immunosuppression caused by leukemia, human immunodeficiency virus infection, phototherapy, and topical calcineurin inhibitors, as being a cause for increased Demodex density or demodicosis.2
In a recent study published in the International Journal of Dermatology,2 researchers Aksoy et al suggest a potential association between psoriasis and increased Demodex density among patients treated with biological therapies.3
They conducted a cross-sectional study between May 2023 and June 2023, enrolling 67 patients with psoriasis who were categorized into those undergoing biologic therapy (n=34) and controls, including individuals receiving topical treatment (n=26) and treatment-naive patients (n=7).
Researchers recorded patients' demographic data, treatment regimens, and presence of associated skin conditions. Certain skin conditions, such as acne, blepharitis, rosacea, folliculitis, perioral dermatitis, pityriasis folliculorum, and seborrheic dermatitis, were noted, as these conditions have been associated with an increase in Demodex density.
Additionally, they assessed Demodex density using the standardized skin surface biopsy technique across 4 facial regions, primarily the cheeks, forehead, and nose. Demodex density increases were defined by the presence of 5 or greater Demodex mites per square centimeter of the skin when examined microscopically.
The analysis of 268 facial samples revealed a statistically significant increase in Demodex density among recipients of biologic therapy compared to controls.
Patients treated with biologic agents showed significantly higher Demodex mite counts per square centimeter on the right cheek, left cheek, and the whole face. The mite count was also numerically higher on the forehead and nose in the biologic therapy group. Specifically, when analyzing Demodex folliculorum density alone, the forehead mite density was significantly higher in the biologic therapy group than in the control group.
Furthermore, certain biologic agents exhibited higher associations with demodicosis. The rates were: 100% for guselkumab, certolizumab, and infliximab; 80% for ixekizumab; 66.7% for risankizumab; 60% for ustekinumab; 50% for secukinumab; and 25% for adalimumab.
Potential study limitations, as noted by researchers, include the study's cross-sectional design and the study's smaller sample size.
"Understanding the relationship between immunosuppression and Demodex colonization could provide valuable insights into the complex interactions between the immune system and commensal microorganisms," wrote study authors Aksoy et al. "Our study contributes to the growing body of evidence linking immunosuppression and Demodex colonization. By shedding light on the association between biologic therapy and Demodex density in psoriasis patients, we provide a basis for further research and potential interventions to minimize the risk of demodicosis in immunosuppressed individuals."
References
- Forton FMN, De Maertelaer V. Which factors influence Demodex proliferation? A retrospective pilot study highlighting a possible role of subtle immune variations and sebaceous gland status. J Dermatol. 2021. Accessed April 25, 2024.
- Herron MD, O'reilly MA, Vanderhooft SL. Refractory Demodex folliculitis in five children with acute lymphoblastic leukemia. Pediatr Dermatol. 2005. Accessed April 25, 2024.
- Aksoy H, Altıntaş Kakşi S, Gönüllü Ö, Aslan Kayıran M, Erdemir VA. Biologic therapy increases Demodex density in psoriasis patients. Int J Dermatol. April 22, 2024. Accessed April 24, 2024. doi:10.1111/ijd.17161
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.