- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
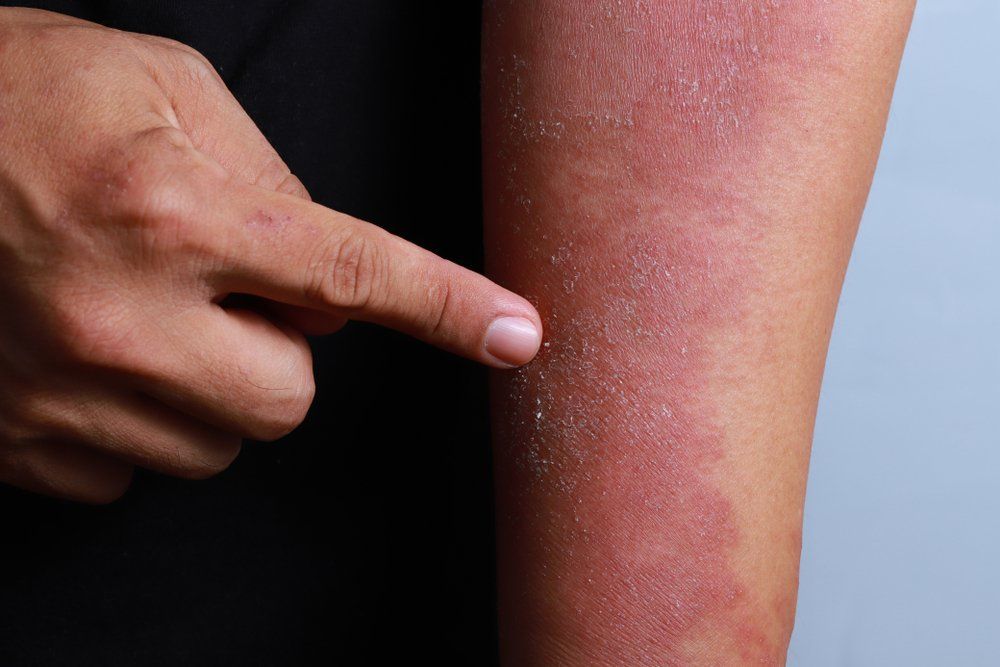
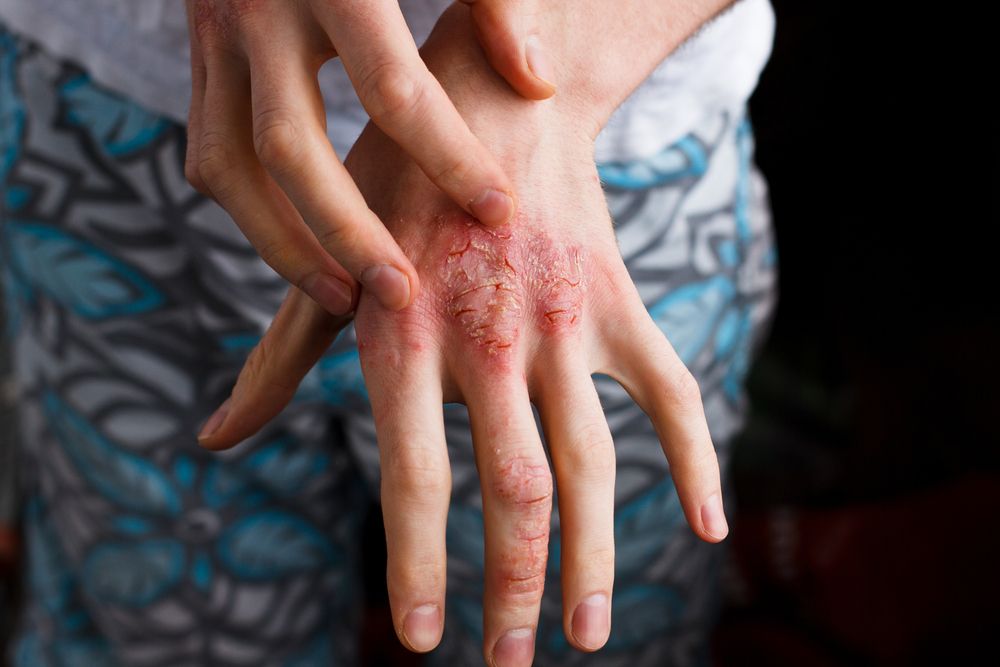
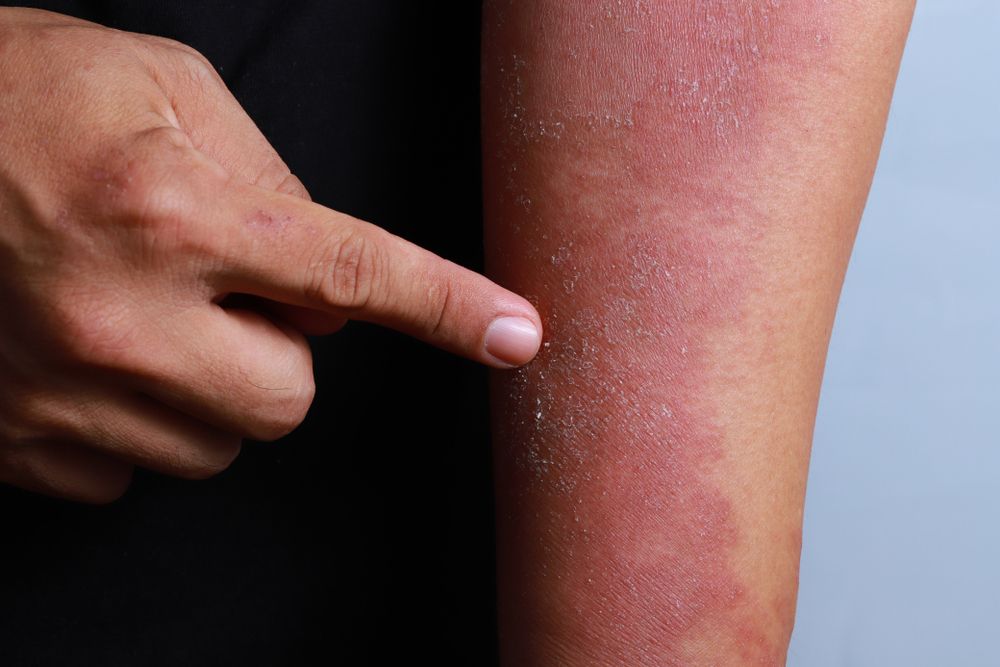
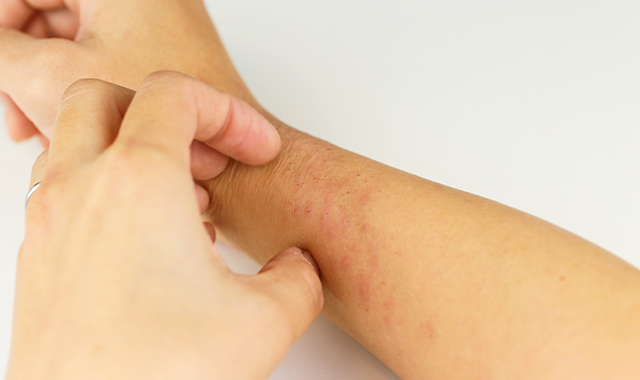
Biologic clears severe dermatitis of the hands
Author(s):
Three patients achieve 80-100 percent clearance of moderate-to-severe dyshidrosis of the hands and palms after treatment with dupilumab.
Dupilumab, the first biologic approved for moderate-to-severe atopic dermatitis in the U.S., should be considered to treat patients with palmar hand eczema, suggests a physician writing in the March 10 issue of the Journal of the American Academy of Dermatology.
Dupilumab (Dupixent, Regeneron Pharmaceuticals) is a human monoclonal antibody against interleukin-4 receptor alpha. Administered as a subcutaneous injection, dupilumab inhibits interleukin-4 and interleukin-13 signaling, which have been shown to be important drivers of atopic dermatitis.
But while dupilumab therapy for atopic dermatitis has been studied in conjunction with corticosteroids, patients in those clinical trials had atopic dermatitis affecting more than 10% of body surface area. When atopic dermatitis is confined to the hands, palms and fingers, it represents only 1% of body surface area.
Despite the existence of multiple etiologies, identifying an individual patient’s etiology often is impossible.
In severe cases, hand eczema can significantly impact quality of life, including having major effects on social interactions, occupational success and daily living activities, says Matthew J. Zirwas, M.D., the article’s author.
Adding to the problem is a lack of consistently effective therapy options for severe hand eczema patients. Mild cases often respond to lifestyle changes, moisturizers and intermittent topical steroid use. But severe disease patients have few if any effective topical or systemic therapy option shown to be effective for atopic dermatitis of the hands.
Dr. Zirwas, of Probity Medical Research in Ohio, described three patients with severe, chronic recalcitrant eczema on the hands and/or arms. The patients were female and male, ages 48 to 72 years, and suffered from hand eczema for nine years or longer. All had been treated with clobetasol under occlusion, as well as with tacrolimus under occlusion with no response. Two tried systemic corticosteroids. Among the other treatments that failed to offer a response: methotrexate, apremilast and ustekinumab.
All three, however, responded to dupilumab, with responses ranging from 80% improvement to complete clearance. One patient achieved complete clearance in six weeks after starting treatment and maintained that for at least three months.
Patients were patch tested prior to receiving dupilumab therapy to rule out relevant allergens. The patients were treated with 600 mg subcutaneously on day 1, followed by 300 mg subcutaneously every 14 days.
“…this is the first report of dupilumab therapy for patients with dermatitis limited primarily to the palms,” the author writes.
REFERENCE:
Zirwas MJ. “Dupilumab for Hand Eczema.” Journal of the American Academy of Dermatology. March 10, 2018. DOI:10.1016/j.jaad.2018.02.073