- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
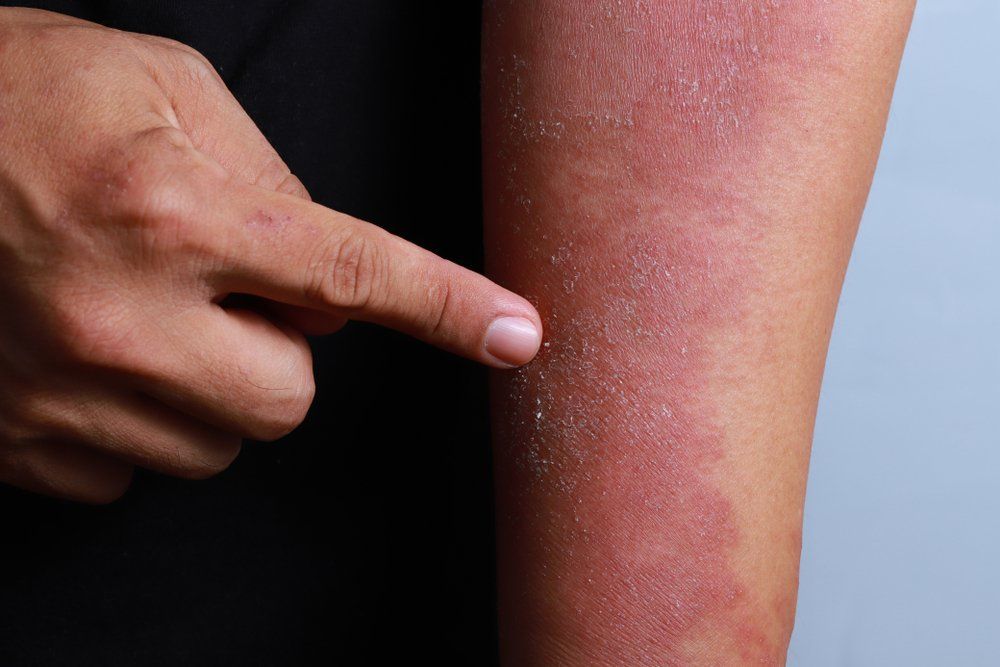
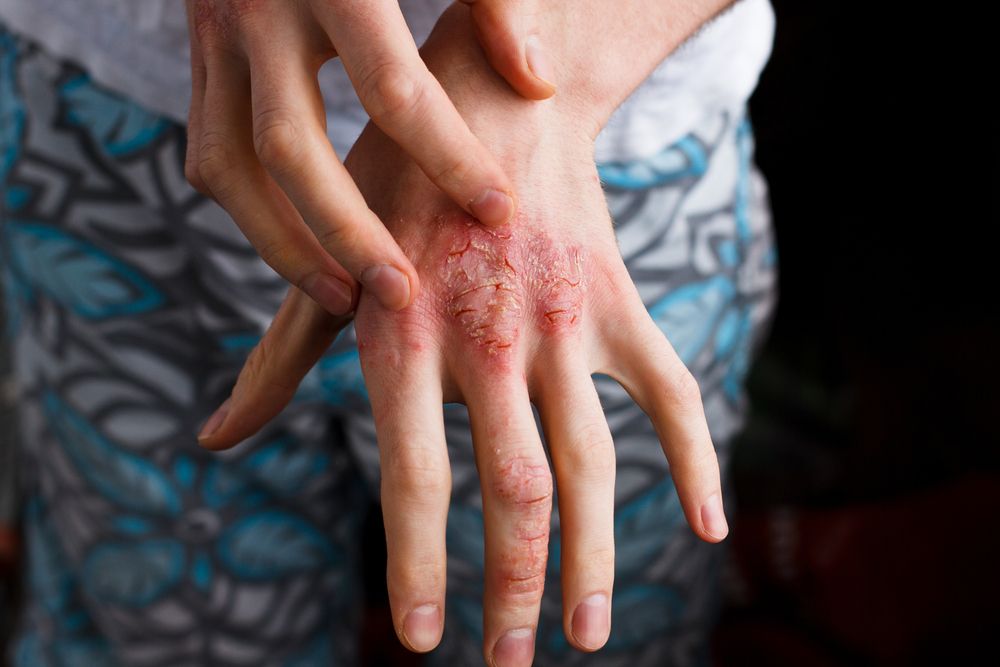
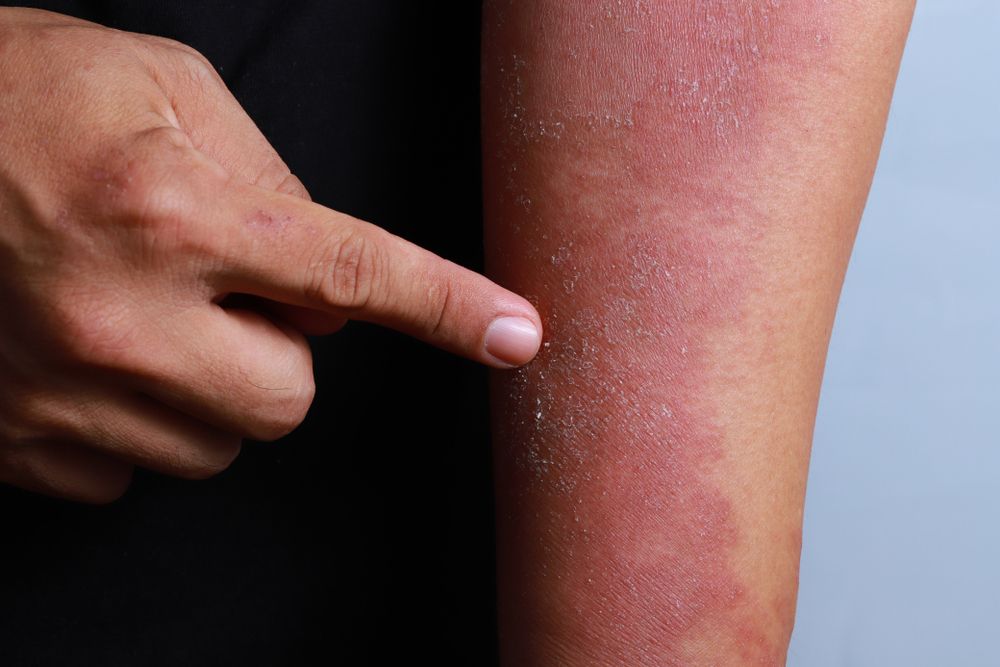
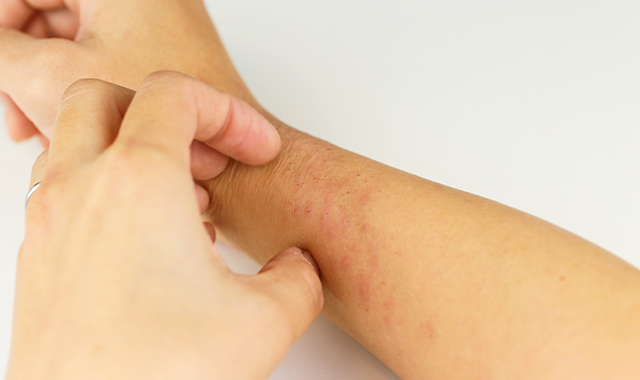
Atopic Dermatitis in 2018
Author(s):
Until recently, atopic dermatitis treatments were primarily limited to topical corticosteroids and systemic immunosuppressants. Now, two new therapies have reached clinical practice. Nineteen more are in development.
Until recently, atopic dermatitis treatments were primarily limited to topical corticosteroids and systemic immunosuppressants.
Now, two new therapies have reached clinical practice, and by one recent account, there are at least 19 more topicals and biologics in development for atopic dermatitis.
“Our improved understanding about the mechanism for development of atopic dermatitis is leading to an expanding pipeline of new and more targeted treatment interventions,” Amy S. Paller, M.D. and colleagues wrote in a review published in September in the Journal of Allergy and Clinical Immunology.
The emerging agents in atopic dermatitis include IL-4, IL-13, IgE, B-cells, IL-5, IL-31, JAK-STAT, SYK, IL-6, PDE-4, IL-12, IL-17, IL-23, IL-22, H4R, NKR1, κOR, TSLP, PPAR-γ, and DGLA. In some cases, clinical trials have shown statistically significant improvements in clinical severity scores and patient-reported outcomes. In other cases, safety profiles are good, but many of these agents are in early trials.
MOST PROMISING AD TREATMENTS
Among the most promising of new treatments are crisaborole (Eucrisa/Pfizer) and dupilumab (Dupixent/Regeneron). Crisaborole is a non-steroidal topical phosphodieterase-4 (PDE-4) inhibitor approved by the U.S. Food and Drug Administration in 2016 for mild to moderate eczema (atopic dermatitis) in patients 2 years old or older. Dupilumab is the first highly effective monoclonal antibody to be approved by the FDA. It targets IL-4/IL-13 and was approved early this year for adults with moderate-to-severe atopic dermatitis.
There are 12 other biologic agents under development, including nemolizumab, an anti-IL-31 receptor that specifically targets a cytokine that causes itch, which, if successful in clinical trials, would be the first biologic agent designed to control itch.
There are six orally administered small-molecule inhibitors that have reached at least phase 2 trials, including baricitinib, an oral JAK1/2 inhibitor that is under study in the United States for rheumatoid arthritis. Researchers reporting at the European Academy of Dermatology and Venereology (EADV) Congress earlier this year, said that baricitinib improved atopic dermatitis symptoms in combination with a topical corticosteroid, and serlopitant - the NK1R inhibitor being studied specifically for pruritus in atopic dermatitis.
These emerging therapies have the potential to revolutionize care, though there could be barriers to optimal clinical practice, including cost.
“The extent of their effect on patient care will depend on their affordability and accessibility to patients with atopic dermatitis from a broad range of socioeconomic groups and with different insurance status,” the authors wrote.
Long-term safety of target therapy is also an open question that requires further research, according to the authors of a review of novel therapeutic approaches to atopic dermatitis published in August in Archivum Immunologiae et Therapiae Experimentalis (AITE).
MORE RESEARCH FOR AD
Atopic dermatitis is entering a new era of target-specific treatments writes Vivian Y. Shi, M.D., and colleagues in a review of new and emerging targeted systemic therapies published in the Journal of Dermatological Treatment.
“While these agents have demonstrated efficacy and potential, only dupilumab has been approved to date for adults with atopic dermatitis,” Dr. Shi and co-authors wrote. “Further large-scale, randomized, placebo-controlled, double-blind, multicenter trials are needed to fully evaluate the clinical benefit, safety, and cost-effectiveness of these emerging therapies.”
A clear gap in the data right now is the role of novel atopic dermatitis treatments in children, which is especially important since atopic dermatitis is more prevalent in pediatric populations, the authors stated.
Further research in novel targeted atopic dermatitis therapies may be advanced by the development of The International Treatment of Atopic Eczema (TREAT) Registry Taskforce, which was started in 2017 and is designed to integrate data on systemic immunomodulatory therapies and phototherapy from dermatology communities around the world.
“The completion and continuous update of this task force will reduce heterogeneity among international clinical trial registries, allow direct comparison of data sets, and facilitate drug efficacy and safety monitoring,” Dr. Shi and colleagues wrote.
REFERENCES
- Paller AS, Kabashima K, Bieber T. “Therapeutic pipeline for atopic dermatitis: End of the drought?”The Journal of Allergy and Clinical Immunology. September 2017. DOI 10.1016/j.jaci.2017.07.006
- Osinka K, Dumycz K, Kwiek B, Feleszko W. “Novel Therapeutic Approaches to Atopic Dermatitis,” Archivum Immunologiae et Therapiae Experimentalis. August 2017. DOI.org/10.1007/s00005-017-0487-1
- Lee DE, Clark AK, Tran KA, Shi VY. “New and Emerging Targeted Systemic Therapies: A New Era for Atopic Dermatitis,”Journal of Dermatological Treatment. Sept. 19, 2017. DOI:10.1080/09546634.2017.1373736