- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
News
Article
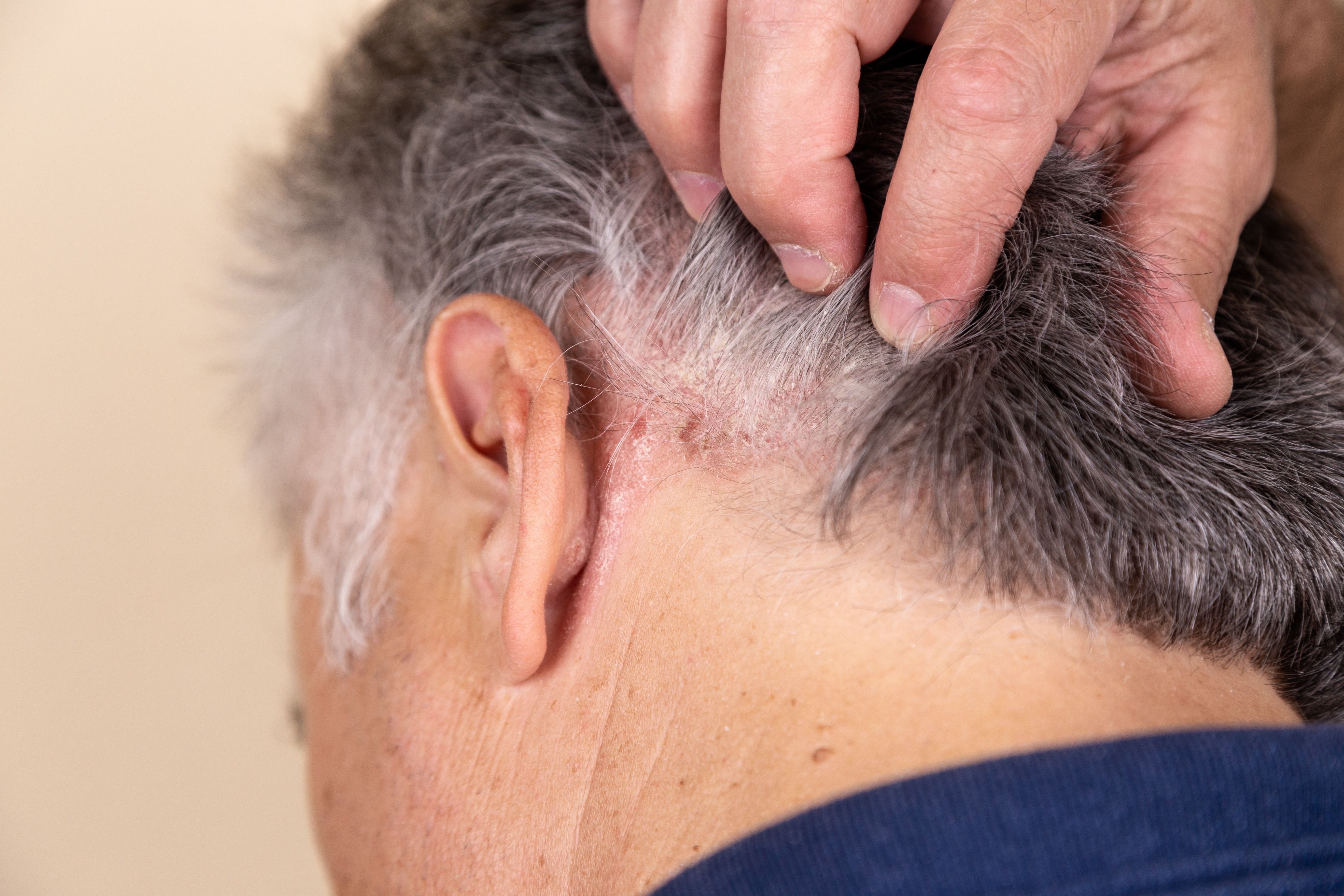
Arcutis Presents Positive Roflumilast Foam Data From ARRECTOR Trial in Scalp and Body PsO at EADV
Author(s):
At week 8, 65.3% of roflumilast-treated patients achieved a clinically significant reduction in itch.
Today, Arcutis Biotherapeutics announced new positive data from its pivotal phase 3 ARRECTOR trial (NCT05028582) of roflumilast foam 0.3% for the treatment of adults and adolescents with scalp and body psoriasis. The results, including new patient-reported outcome data, were presented at the European Academy of Dermatology and Venereology (EADV) Congress in Berlin, Germany.1
Key findings from the study include:
- 65.3% of roflumilast-treated patients achieved a clinically significant reduction in itch compared to 30.3% of vehicle-treated patients at week 8 (P<0.0001), measured by a 4-point change from baseline in Scalp Itch-Numeric Rating Score (SI-NRS)
- Rapid and significant improvement in scalp itch was seen 24 hours following the first application, measured by SI-NRS (P=0.0164)
- Improvement in body itch, measured by the Worst Itch Numeric Rating Scale (WI-NRS), was also observed at week 8, with 63.1% of roflumilast-treated patients achieving a WI-NRS 4-point response compared to 30.1% of patients treated with vehicle (P<0.0001)
According to the study, patient-reported outcomes, reported by the Psoriasis Symptom Diary (PSD), also improved. Patients were given a 16-item PSD questionnaire to assess efficacy by asking them to rate the severity and burden of their psoriasis-related symptoms in the past 24 hours. Findings from the questionnaire include:
- At week 8, a statistically significantly greater percentage of patients achieved an aggregate PSD score of 0 (ie, no symptoms) for scaling, itch, and pain, with 19.6% of roflumilast-treated patients reporting no scaling, itch, or pain compared to 7.1% of those treated with vehicle (P=0.0012)
- Regarding the severity of scaling, 41.5% of roflumilast-treated patients achieved a PSD score of 0 vs. 13.6% of vehicle-treated patients (P<0.0001)
- For the severity of itch, 31.7% of roflumilast-treated patients achieved a PSD score of 0 vs. 10.0% of vehicle-treated patients (P<0.0001)
- Regarding the severity of pain, 64.9% of roflumilast-treated patients achieved a PSD score of 0 compared to 40.3% of individuals treated with vehicle (P<0.0001)
In total, 432 adults and adolescents ages 12 and over were enrolled in ARRECTOR. The extent of scalp involvement of patients was approximately one-third of the scalp surface area (34.4% for patients treated with roflumilast foam, 36.0% for patients treated with a vehicle foam). The previous use of a topical steroid was reported by 81.9% of patients, and 58.6% of patients reported inadequate response, intolerance, or contraindication to topical steroids.
“These data from the ARRECTOR trial show once-daily topical roflumilast foam achieved early and significant improvements in psoriasis signs and symptoms on both the scalp and body, including in patients who have already failed standard-of-care topical steroids, suggesting investigational roflumilast foam may be an important topical treatment option,” said Patrick Burnett, MD, PhD, FAAD, chief medical officer of Arcutis, in the news release. “We are pleased to share these results with the medical dermatology community at EADV as we further explore the potential of roflumilast foam in patients with psoriasis in hair-bearing areas of the body, which are traditionally challenging to treat.”
Reference
- Arcutis presents positive patient-reported outcome data from the pivotal ARRECTOR phase 3 trial in scalp and body psoriasis at European Academy of Dermatology and Venereology (EADV) Congress. Arcutis Biotherapeutics. News release. October 13, 2023. Accessed October 13, 2023. https://www.arcutis.com/arcutis-presents-positive-patient-reported-outcome-data-from-the-pivotal-arrector-phase-3-trial-in-scalp-and-body-psoriasis-at-european-academy-of-dermatology-and-venereology-eadv-congress/