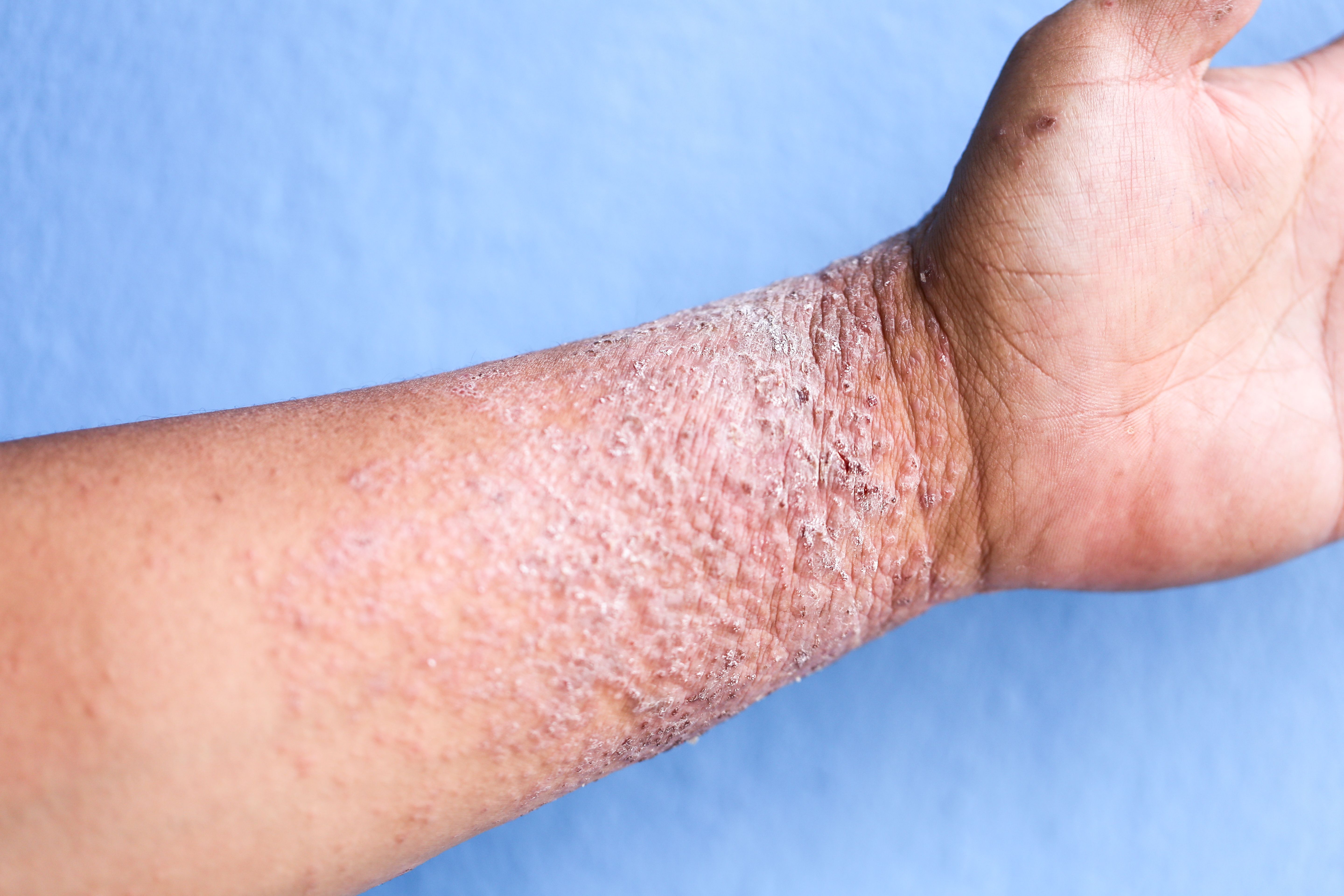
Almirall has announced1 the presentation of new data centered around the clinical efficacy, improvements, and meaningful responses associated with lebrikizumab treatment in patients with atopic dermatitis (AD).
The data, presented at the European Academy of Dermatology and Venereology (EADV) Congress, supports the ability of treatment with lebrikizumab to lead to clinically meaningful patient responses.
Among the 18 abstracts and presentations presented by Almirall at EADV was new data stemming from the phase 3 ADvantage study. The data revealed that patients with moderate-to-severe AD who had previously were ineligible for treatment with cyclosporine, or who had inadequately controlled AD as a result of cyclosporine use, demonstrated clinical improvements when treated with a combination of lebrikizumab and a topical corticosteroid over a 16-week duration.
Key Takeaways
- Lebrikizumab treatment for atopic dermatitis (AD) demonstrates clinical improvements in patients previously ineligible for or inadequately controlled with cyclosporine, according to data from the phase 3 ADvantage study.
- Phase 3 monotherapy trials ADvocate 1 and ADvocate 2 show that lebrikizumab offers sustained and deep responses in AD patients, with 20% and 31% achieving total skin clearance by week 16, and a significant proportion achieving mild or clear/almost clear skin.
“Atopic dermatitis (AD) is a debilitating chronic skin condition that can be challenging to manage. Cyclosporine A is the only classical systemic treatment approved in Europe for AD, but its safety may limit long-term use, or it may be contraindicated for some patients”, said Ricard B. Warren, MD, in a press release1 from Almirall. Warren is a professor of dermatology and honorary consultant dermatologist at the University of Manchester and Northern Care Aliance NHS Foundation Trust, as well as the principal investigator of the ADvantage trial.
“Although there are an increasing number of treatment options available to ease symptoms and improve outcomes of AD, unfortunately there are few that offer long-term disease control with a favorable safety profile," Warren said. "The results of this trial reinforce our confidence that lebrikizumab is a promising potential new treatment for patients with moderate-to-severe AD, including those not adequately controlled or ineligible for treatment with cyclosporine A.”
Additionally, data from the phase 3 monotherapy trials ADvocate 1 and ADvocate 2 showed that over the course of 52 weeks, patients achieved a sustained depth of response when treated with lebrikizumab. By week 16, 20% and 31% of patients had achieved total skin clearance. This deep response was maintained or improved upon through week 52.
A post-hoc analysis of the ADvocate studies showed that compared to patients treated with a placebo, a significantly higher proportion of lebrikizumab-treated patients achieved an EASI score equal to or less than 7 (mild) or 1 (clear/almost clear) by week 16. Furthermore, more than half of patients treated with lebrikizumab achieved an EASI score indicative of mild AD, while 20% exhibited EASI scores indicative of clear or almost clear skin.
“The new lebrikizumab data set presented at the EADV congress provides further evidence of its efficacy and safety profile. Its anticipated addition to the moderate-to-severe AD treatment arsenal is promising news for both healthcare professionals and patients, including those who do not respond adequately to cyclosporine," said Karl Ziegelbauer, PhD, chief scientific officer of Almirall.1 "Our teams are diligently working to increase our level of knowledge and expand the body of evidence, thereby reinforcing the profile of this potential first-line treatment."
Reference
- Almirall’s lebrikizumab improves signs and symptoms of moderate-to-severe atopic dermatitis (AD) in patients inadequately controlled with or ineligible for cyclosporine. Almirall. October 13, 2023. Accessed October 13, 2023. https://www.almirall.com/newsroom/news/almiralls-lebrikizumab-improves-signs-and-symptoms-of-moderate-to-severe-atopic-dermatitis-ad-in-patients-inadequately-controlled-with-or-ineligible-for-cyclosporine